Sections
Section I: Genotype Summary
Genotypes called: 18 / 19
| Drugs | Gene |
Genotypes
|
||||||
|---|---|---|---|---|---|---|---|---|
| ABCG2 ‡ |
|
|||||||
| CACNA1S † ‡ |
|
|||||||
| CFTR ‡ |
|
|||||||
| CYP2B6 † ‡ |
|
|||||||
| CYP2C19 ‡ |
|
|||||||
|
|
CYP2C9 † ‡ |
|
||||||
| CYP3A4 † ‡ |
|
|||||||
| CYP3A5 † ‡ |
|
|||||||
| CYP4F2 † ‡ |
|
|||||||
| DPYD † |
|
|||||||
|
|
G6PD † ‡ |
|
||||||
|
|
IFNL3/4 ‡ |
|
||||||
| NUDT15 † ‡ |
|
|||||||
| RYR1 † |
|
|||||||
|
|
SLCO1B1 † ‡ |
|
||||||
| TPMT † ‡ |
|
|||||||
|
|
UGT1A1 † ‡ |
|
||||||
| VKORC1 ‡ |
|
|||||||
Section II: Prescribing Recommendations
abrocitinib
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
"In patients who are known or suspected to be CYP2C19 poor metabolizers, the recommended dosage of CIBINQO [abrocitinib] is 50 mg once daily. If an adequate response is not achieved with CIBINQO 50 mg orally daily after 12 weeks, consider increasing dosage to 100 mg orally once daily. Discontinue therapy if inadequate response is seen after dosage increase to 100 mg once daily." See label for more information. * | Unspecified | |
|
Affected subgroup: CYP2C19 poor metabolizers Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations and may result in higher adverse reaction risk. Dosage adjustment is recommended. Refer to FDA labeling for specific dosing recommendations." * | Unspecified |
acenocoumarol
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
-1639 GG |
The guideline does not provide a description of the impact of the -1639 GG genotype on acenocoumarol. | The guideline does not provide a recommendation for acenocoumarol in patients with the VKORC1 rs9923231 CC genotype (-1639 GG genotype). | No recommendation |
Citations:
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
allopurinol
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Function |
The guideline does not provide a description of the impact of the ABCG2 rs2231142 GG genotype (c.421CC; p.141QQ) on allopurinol. | The guideline does not provide a recommendation for allopurinol in patients with the the ABCG2 rs2231142 GG genotype (c.421CC; p.141QQ) | No recommendation |
Citations:
- Dutch pharmacogenetics working group guideline for the gene-drug interaction of ABCG2, HLA-B and Allopurinol, and MTHFR, folic acid and methotrexate. European journal of human genetics : EJHG. 2024. PMID:36056234
amitriptyline
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Alternate Drug
Dosing Info
|
Genotype
Phenotypes
Activity Scores
|
|
Avoid tertiary amine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include the secondary amines nortriptyline and desipramine. For tertiary amines, consider a 50% reduction of the recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects.
Other ConsiderationsDosing recommendations only apply to higher initial doses of TCAs for treatment of conditions such as depression. Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects. Patients may receive an initial low dose of a tricyclic, which is then increased over several days to the recommended steady-state dose. The starting dose in this guideline refers to the recommended steady-state dose. |
Moderate |
Citations:
- Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clinical pharmacology and therapeutics. 2013. PMID:23486447
- Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clinical pharmacology and therapeutics. 2017. PMID:27997040
atazanavir
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotype
Normal Metabolizer |
UGT1A1: Reference UGT1A1 activity; very low likelihood of bilirubin-related discontinuation of atazanavir. |
There is no need to avoid prescribing of atazanavir based on UGT1A1 genetic test result. Inform the patient that some patients stop atazanavir because of jaundice (yellow eyes and skin), but that this patient’s genotype makes this unlikely (less than about a 1 in 20 chance of stopping atazanavir because of jaundice).
Other ConsiderationsAll studies correlating UGT1A1 genotypes with atazanavir adverse events have involved ritonavir boosting. However, concentration-time profiles are equivalent when boosted with either cobicistat or ritonavir (PMID 23532097), and bilirubin-related adverse events including discontinuation of atazanavir occur in a similar percentage of patients prescribed atazanavir with cobicistat or ritonavir (PMID 23532097). Associations between UGT1A1 genotype, bilirubin elevations, and atazanavir/r discontinuation therefore almost certainly translate to atazanavir/cobicistat. "reference" function refers to the UGT1A1 allele to which other alleles are compared. |
Strong |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for UGT1A1 and Atazanavir Prescribing. Clinical pharmacology and therapeutics. 2016. PMID:26417955
atorvastatin
In case no genotype can be determined, recommendations are based on the rs4149056 genotype alone as per guideline. The minor C allele at rs4149056 defines SLCO1B1*5.
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Function |
SLCO1B1: Typical myopathy risk and statin exposure |
Prescribe desired starting dose and adjust doses based on disease-specific guidelines.
Other ConsiderationsThe potential for drug-drug interactions and dose limits based on renal and hepatic function and ancestry should be evaluated prior to initiating a statin. |
Strong | |
|
Population: No Action
|
Genotype
Phenotype
Normal Function |
The guideline does not provide a description of the impact of the _SLCO1B1_ 521 TT genotype on atorvastatin. | The guideline does not provide a recommendation for atorvastatin in patients with the SLCO1B1 521 TT genotype | No recommendation | |
|
FDA PGx Association 1 |
Genotype
SLCO1B1:*1/*1
|
|
|||
Citations:
- The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 genotypes and Statin-Associated Musculoskeletal Symptoms. Clinical pharmacology and therapeutics. 2022. PMID:35152405
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between SLCO1B1 and statins and CYP2C9 and sulfonylureas. European journal of human genetics : EJHG. 2024. PMID:39676086
- FDA Table of Pharmacogenetic Associations.
azathioprine
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotypes
|
|
Start with normal starting dose (e.g., 2-3 mg/kg/day) and adjust doses of azathioprine based on disease-specific guidelines. Allow 2 weeks to reach steady-state after each dose adjustment (PMID 20354201, 11302950, 15606506).
Other ConsiderationsNormal starting doses vary by race/ethnicity and treatment regimens. If standard dose is below normal recommended dose, dose reduction might not be recommended for intermediate metabolizers. |
Strong | |
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on azathioprine. | The guideline does not provide a recommendation for azathioprine in normal metabolizers | No recommendation | |
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on azathioprine. | The guideline does not provide a recommendation for azathioprine in normal metabolizers. | No recommendation | |
|
FDA Label Annotation 1 |
Genotypes
NUDT15:*1/*1;TPMT:*1/*1 |
|
|||
|
FDA PGx Association 1 |
Genotypes
NUDT15:*1/*1;TPMT:*1/*1 |
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clinical pharmacology and therapeutics. 2011. PMID:21270794
- Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clinical pharmacology and therapeutics. 2013. PMID:23422873
- Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clinical pharmacology and therapeutics. 2019. PMID:30447069
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Drugs@FDA: Drug Product IMURAN (azathioprine), NDA016324, Sebela Pharmaceuticals Inc.
- FDA Table of Pharmacogenetic Associations.
belzutifan
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotype
Poor Metabolizer |
"Patients who are dual UGT2B17 and CYP2C19 poor metabolizers have higher belzutifan exposures, which may increase the incidence and severity of adverse reactions of WELIREG [belzutifan]." | "Closely monitor for adverse reactions in patients who are dual UGT2B17 and CYP2C19 poor metabolizers." See label for more information. * | Unspecified |
|
Affected subgroup: CYP2C19 and/or UGT2B17 poor metabolizers Other Guidance
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations and may result in higher adverse reaction risk (anemia, hypoxia). Monitor patients who are poor metabolizers for both genes for adverse reactions." * | Unspecified |
brivaracetam
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
The brivaracetam (BRIVIACT) label states: "CYP2C19 poor metabolizers and patients using inhibitors of CYP2C19 may require dose reduction." See label for more information. * | Unspecified | |
|
Affected subgroup: CYP2C19 intermediate or poor metabolizers Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations and higher adverse reaction risk. Consider dosage reductions in poor metabolizers." * | Unspecified |
capecitabine
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Score
2.0 |
DPYD: Normal DPD activity and "normal" risk for fluoropyrimidine toxicity | Based on genotype, there is no indication to change dose or therapy. Use label-recommended dosage and administration. | Strong | |
|
Population: No Action
|
Genotype
Phenotype
2.0 (Normal Metabolizer) |
The guideline does not provide a description of the impact of a DPYD activity score of 2 on capecitabine. | The guideline does not provide a recommendation for capecitabine in patients with a DPYD activity score of 2. | No recommendation | |
|
FDA Label Annotation 1 |
Genotype
DPYD:Reference/Reference
|
|
|||
|
FDA PGx Association 1 |
Genotype
DPYD:Reference/Reference
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clinical pharmacology and therapeutics. 2013. PMID:23988873
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clinical pharmacology and therapeutics. 2018. PMID:29152729
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction of DPYD and fluoropyrimidines. European journal of human genetics : EJHG. 2020. PMID:31745289
- Drugs@FDA: Drug Product Xeloda (capecitabine), NDA020896, Genentech, Inc.
- FDA Table of Pharmacogenetic Associations.
carisoprodol
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotype
Poor Metabolizer |
"Patients with Reduced CYP2C19 Activity: SOMA [carisoprodol] should be used with caution in patients with reduced CYP2C19 activity." See label for more information. * | Unspecified | |
|
Affected subgroup: CYP2C19 poor metabolizers Other Guidance
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations. Use with caution." * | Unspecified |
celecoxib
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Score
2.0 |
CYP2C9: Normal metabolism | Initiate therapy with recommended starting dose. In accordance with the prescribing information, use the lowest effective dosage for shortest duration consistent with individual patient treatment goals. | Strong | |
|
FDA Label Annotation 1 |
Genotype
CYP2C9:*1/*1
|
|
|||
|
FDA PGx Association 1 |
Genotype
CYP2C9:*1/*1
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clinical pharmacology and therapeutics. 2020. PMID:32189324
- Drugs@FDA: Drug Product CELEBREX (Celecoxib), NDA020998, Aphena Pharma Solutions - Tennessee, LLC.
- FDA Table of Pharmacogenetic Associations.
citalopram
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Alternate Drug
Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Reduced metabolism of citalopram and escitalopram to less active compounds when compared to CYP2C19 normal and intermediate metabolizers. Higher plasma concentrations may increase the probability of side effects. |
Consider a clinically appropriate antidepressant not predominantly metabolized by CYP2C19. If citalopram or escitalopram are clinically appropriate, consider a lower starting dose, slower titration schedule and 50% reduction of the standard maintenance dose as compared to normal metabolizers.
Other ConsiderationsPer the FDA warning, citalopram 20 mg/day is the maximum recommended dose in CYP2C19 poor metabolizers due to the risk of QT prolongation. FDA product labeling additionally cautions that citalopram dose should be limited to 20 mg/day in patients with hepatic impairment, those taking a CYP2C19 inhibitor, and patients greater than 60 years of age. |
Strong |
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
The risk of QT prolongation and therefore also the theoretical risk of torsades de pointes is increased as the gene variation leads to an increased citalopram plasma concentration. If you follow the dose recommendation below, the increased plasma concentration and the increased risk of QT prolongation will be offset. |
Do not exceed the following daily doses (50% of the standard maximum dose):
|
Unspecified |
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19 poor metabolizers have higher systemic concentration and adverse reaction risk (QT prolongation). | "Celexa [citalopram] 20 mg/day is the maximum recommended dose in CYP2C19 poor metabolizers due to the risk of QT prolongation." See label for more information. * | Unspecified |
|
Affected subgroup: CYP2C19 poor metabolizers Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations and adverse reaction risk (QT prolongation). The maximum recommended dose is 20 mg." * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clinical pharmacology and therapeutics. 2015. PMID:25974703
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clinical pharmacology and therapeutics. 2023. PMID:37032427
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. European journal of human genetics : EJHG. 2022. PMID:34782755
- Drugs@FDA: Drug Product Celexa (citalopram), NDA020822, Allergan, Inc.
- FDA Table of Pharmacogenetic Associations.
clobazam
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
"In CYP2C19 poor metabolizers ... the starting dose should be 5 mg/day and dose titration should proceed slowly according to weight, but to half the dose presented in Table 1 [of the drug label], as tolerated. If necessary and based upon clinical response, an additional titration to the maximum dose (20 mg/day or 40 mg/day, depending on the weight group) may be started on day 21." See label for more information. * | Unspecified | |
|
Affected subgroup: CYP2C9 intermediate or poor metabolizers Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic active metabolite concentrations. Poor metabolism results in higher adverse reaction risk. Dosage adjustment is recommended. Refer to FDA labeling for specific dosing recommendations." * | Unspecified |
clomipramine
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Alternate Drug
Dosing Info
|
Genotype
Phenotypes
Activity Scores
|
|
Avoid tertiary amine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include the secondary amines nortriptyline and desipramine. For tertiary amines, consider a 50% reduction of the recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects.
Other ConsiderationsDosing recommendations only apply to higher initial doses of TCAs for treatment of conditions such as depression. Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects. Patients may receive an initial low dose of a tricyclic, which is then increased over several days to the recommended steady-state dose. The starting dose in this guideline refers to the recommended steady-state dose. |
Optional |
|
Population: No Action
|
Genotype
Phenotype
Poor Metabolizer |
The gene variation increases the plasma concentration of clomipramine. However, there is insufficient evidence to substantiate an increase of the plasma concentration of clomipramine+desmethylclomipramine to such an extent that it increases the risk of side effects. The increase in the plasma concentration of clomipramine is favourable for the efficacy in anxiety and obsessive compulsive disorder. | NO action is required for this gene-drug interaction. | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clinical pharmacology and therapeutics. 2013. PMID:23486447
- Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clinical pharmacology and therapeutics. 2017. PMID:27997040
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
clopidogrel
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Alternate Drug
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Significantly reduced clopidogrel active metabolite formation; increased on-treatment platelet reactivity; increased risk for adverse cardiac and cerebrovascular events |
Avoid clopidogrel if possible. Use prasugrel or ticagrelor at standard dose if no contraindication.
Other ConsiderationsFor cardiovascular indications of acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI). ACS and/or PCI includes patients undergoing PCI for an ACS or non-ACS (elective) indication. |
Strong |
|
Population: Alternate Drug
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Significantly reduced clopidogrel active metabolite formation; increased on-treatment platelet reactivity; increased risk for adverse cardiac and cerebrovascular events |
Avoid clopidogrel if possible. Use prasugrel or ticagrelor at standard dose if no contraindication.
Other ConsiderationsFor non-acute coronary syndrome (non-ACS) and non-percutaneous coronary intervention (non-PCI) cardiovascular indications. Non-ACS, non-PCI cardiovascular indications include peripheral arterial disease and stable coronary artery disease following a recent myocardial infarction outside the setting of PCI. |
Moderate |
|
Population: Alternate Drug
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Significantly reduced clopidogrel active metabolite formation; increased on-treatment platelet reactivity; increased risk for adverse cardiac and cerebrovascular events |
Avoid clopidogrel if possible. Consider an alternative P2Y12 inhibitor at standard dose if clinically indicated and no contraindication.
Other ConsiderationsFor neurovascular indications. Neurovascular disease includes acute ischemic stroke or transient ischemic attack, secondary prevention of stroke, or prevention of thromboembolic events following neurointerventional procedures such as carotid artery stenting and stent-assisted coiling of intracranial aneurysms. Alternative P2Y12 inhibitors not impacted by CYP2C19 genetic variants include ticagrelor and ticlopidine. Prasugrel is contraindicated in patients with a history of stroke or TIA. Given limited outcomes data for genotype-guided anti-platelet therapy for neurovascular indications, selection of therapy should depend on individual patient treatment goals and risks for adverse events. |
Moderate |
|
Population: Alternate Drug
|
Genotype
Phenotype
Poor Metabolizer |
The risk of serious cardiovascular and cerebrovascular events is increased in patients undergoing balloon angioplasty or stent placement (percutaneous coronary intervention) and in patients with a stroke or TIA, because the genetic variation reduces the activation of clopidogrel. No negative clinical consequences have been proved in other patients. | PERCUTANEOUS CORONARY INTERVENTION, STROKE or TIA: avoid clopidogrel. Prasugrel, ticagrelor and acetylsalicylic acid/dipyridamole are not metabolised by CYP2C19 (or to a lesser extent). OTHER INDICATIONS: determine the level of inhibition of platelet aggregation by clopidogrel. Consider an alternative in poor responders. Prasugrel and ticagrelor are not metabolised by CYP2C19 (or to a lesser extent). | Unspecified |
|
Population: Alternate Drug
|
Genotype
Phenotype
Poor Metabolizer |
The clopidogrel (Plavix) label states: "Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers." See label for more information. * | Unspecified | |
|
Affected subgroup: CYP2C19 intermediate or poor metabolizers Alternate Drug
|
Genotype
Phenotype
Poor Metabolizer |
"Results in lower systemic active metabolite concentrations, lower antiplatelet response, and may result in higher cardiovascular risk. Consider use of another platelet P2Y12 inhibitor." * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clinical pharmacology and therapeutics. 2011. PMID:21716271
- Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clinical pharmacology and therapeutics. 2013. PMID:23698643
- Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clinical pharmacology and therapeutics. 2022. PMID:35034351
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Drugs@FDA: Drug Product Plavix (clopidogrel bisulfate), NDA020839, Rebel Distributors Corp.
- Drugs@FDA: Drug Product Plavix (clopidogrel), sanofi-aventis U.S. LLC - NDA020839/SUPPL-78, 09/16/2022.
- FDA Table of Pharmacogenetic Associations.
dapsone
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal |
G6PD: Low risk of acute hemolytic anemia | No reason to avoid based on G6PD status | Strong | |
|
FDA Label Annotation 1 |
Genotype
G6PD:B (reference)/B (reference)
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clinical pharmacology and therapeutics. 2014. PMID:24787449
- Expanded Clinical Pharmacogenetics Implementation Consortium Guideline for Medication Use in the Context of G6PD Genotype. Clinical pharmacology and therapeutics. 2023. PMID:36049896
- Drugs@FDA: Drug Product DAPSONE (dapsone), Pacific Pharma, Inc. - NDA021794/SUPPL-16, 05/18/2018.
desflurane
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotypes
|
These results do not eliminate the chance that this patient is susceptible to malignant hyperthermia (MH). The genetic cause of about half of all MH survivors, with MH susceptibility confirmed by contracture test, remains unknown (PMID 28902675). | Clinical findings, family history, further genetic testing and other laboratory data should guide use of halogenated volatile anesthetics or depolarizing muscle relaxants. | Strong |
|
Population: Alternate Drug
|
Genotype
Phenotypes
|
"The use of SUPRANE [desflurane] is contraindicated in...[cases of k]nown or suspected genetic susceptibility to malignant hyperthermia...SUPRANE [desflurane] can induce malignant hyperthermia in patients with known or suspected susceptibility based on genetic factors or family history, including those with certain inherited ryanodine receptor (RYR1) or dihydropyridine receptor (CACNA1S) variants. " See label for more information. * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for the Use of Potent Volatile Anesthetic Agents and Succinylcholine in the Context of RYR1 or CACNA1S Genotypes. Clinical pharmacology and therapeutics. 2019. PMID:30499100
- Drugs@FDA: Drug Product SUPRANE (desflurane), NDA020118, Baxter Healthcare Corporation.
dexlansoprazole
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Increased plasma concentration of PPI compared to CYP2C19 NMs; increased chance of efficacy and potentially toxicity | Initiate standard starting daily dose. For chronic therapy (>12 weeks) and efficacy achieved, consider 50% reduction in daily dose and monitor for continued efficacy. | Optional |
|
Affected subgroup: CYP2C19 intermediate or poor metabolizers No Action
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations." * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clinical pharmacology and therapeutics. 2021. PMID:32770672
- FDA Table of Pharmacogenetic Associations.
diazepam
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Affected subgroup: CYP2C19 poor metabolizers No Action
|
Genotype
Phenotype
Poor Metabolizer |
"May affect systemic concentrations." * | Unspecified |
Citations:
doxepin
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Alternate Drug
Dosing Info
|
Genotype
Phenotypes
Activity Scores
|
|
Avoid tertiary amine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include the secondary amines nortriptyline and desipramine. For tertiary amines, consider a 50% reduction of the recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects.
Other ConsiderationsDosing recommendations only apply to higher initial doses of TCAs for treatment of conditions such as depression. Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects. Patients may receive an initial low dose of a tricyclic, which is then increased over several days to the recommended steady-state dose. The starting dose in this guideline refers to the recommended steady-state dose. |
Optional |
|
Affected subgroup: CYP2C19 intermediate or poor metabolizers No Action
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations." * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clinical pharmacology and therapeutics. 2013. PMID:23486447
- Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clinical pharmacology and therapeutics. 2017. PMID:27997040
- FDA Table of Pharmacogenetic Associations.
efavirenz
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: Dosing Info
|
Genotype
Phenotype
Intermediate Metabolizer |
CYP2B6: Higher dose-adjusted trough concentrations of efavirenz compared with normal metabolizers; increased risk of CNS adverse events |
Consider initiating efavirenz with decreased dose of 400 mg/day
Other ConsiderationsIf therapeutic drug monitoring is available and a decreased efavirenz dose is prescribed, consider obtaining steady-state plasma efavirenz concentrations to ensure concentrations are in the suggested therapeutic range (~1 to 4 μg/mL). To prescribe efavirenz at a decreased dose of 400 mg/day or 200 mg/day in a multidrug regimen may require prescribing more than one pill once daily. If so, the provider should weigh the potential benefit of reduced dose against the potential detrimental impact of increased pill number. |
Moderate | |
|
Population: Dosing Info
|
Genotype
Phenotype
Intermediate Metabolizer |
Genetic variations increase the efavirenz plasma concentration and therefore the risk of side effects. However, the efavirenz plasma concentration remains within the therapeutic range for the majority of patients. | Determine the efavirenz plasma concentration if side effects occur and reduce the dose if needed. In 14 IM adults, a dose reduction to 400 mg/day (2/3rd of the standard dose) was sufficient to achieve therapeutic plasma concentrations and to reduce or resolve side effects. The therapeutic range established for efavirenz is 1000-4000 ng/ml. | Unspecified | |
|
FDA PGx Association 1 |
Genotype
CYP2B6:*1/*6
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2B6 and Efavirenz-Containing Antiretroviral Therapy. Clinical pharmacology and therapeutics. 2019. PMID:31006110
- FDA Table of Pharmacogenetic Associations.
enflurane
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotypes
|
These results do not eliminate the chance that this patient is susceptible to malignant hyperthermia (MH). The genetic cause of about half of all MH survivors, with MH susceptibility confirmed by contracture test, remains unknown (PMID 28902675). | Clinical findings, family history, further genetic testing and other laboratory data should guide use of halogenated volatile anesthetics or depolarizing muscle relaxants. | Strong |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for the Use of Potent Volatile Anesthetic Agents and Succinylcholine in the Context of RYR1 or CACNA1S Genotypes. Clinical pharmacology and therapeutics. 2019. PMID:30499100
escitalopram
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Alternate Drug
Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Reduced metabolism of citalopram and escitalopram to less active compounds when compared to CYP2C19 normal and intermediate metabolizers. Higher plasma concentrations may increase the probability of side effects. |
Consider a clinically appropriate antidepressant not predominantly metabolized by CYP2C19. If citalopram or escitalopram are clinically appropriate, consider a lower starting dose, slower titration schedule and 50% reduction of the standard maintenance dose as compared to normal metabolizers.
Other ConsiderationsPer the FDA warning, citalopram 20 mg/day is the maximum recommended dose in CYP2C19 poor metabolizers due to the risk of QT prolongation. FDA product labeling additionally cautions that citalopram dose should be limited to 20 mg/day in patients with hepatic impairment, those taking a CYP2C19 inhibitor, and patients greater than 60 years of age. |
Strong |
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
The risk of switching to another antidepressant is increased. In addition, the risk of QT prolongation and torsades de pointes is theoretically increased because the gene variation leads to an increased escitalopram plasma concentration. If you follow the dose recommendation below, the increased plasma concentration, the theoretically increased risk of QT prolongation and the increased risk of switching to another antidepressant will be offset. | Do not exceed the following doses (50% of the standard maximum dose): adults < 65 years 10 mg/day, =65 years 5 mg/day | Unspecified |
|
Affected subgroup: CYP2C19 ultrarapid, intermediate, or poor metabolizers No Action
|
Genotype
Phenotype
Poor Metabolizer |
"May alter systemic concentrations." * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clinical pharmacology and therapeutics. 2015. PMID:25974703
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clinical pharmacology and therapeutics. 2023. PMID:37032427
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. European journal of human genetics : EJHG. 2022. PMID:34782755
- FDA Table of Pharmacogenetic Associations.
esomeprazole
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Affected subgroup: CYP2C19 poor metabolizers No Action
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations." * | Unspecified |
Citations:
flibanserin
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotype
Poor Metabolizer |
The flibanserin (ADDYI) label states: "[...]increase monitoring for adverse reactions (e.g., hypotension) in patients who are CYP2C19 poor metabolizers." See label for more information. * | Unspecified | |
|
Affected subgroup: CYP2C19 poor metabolizers Other Guidance
|
Genotype
Phenotype
Poor Metabolizer |
"May result in higher systemic concentrations and higher adverse reaction risk. Monitor patients for adverse reactions." * | Unspecified |
flucytosine
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
2.0 (Normal Metabolizer) |
The guideline does not provide a description of the impact of a DPYD activity score of 2 on flucytosine. | The guideline does not provide a recommendation for flucytosine in patients with a DPYD activity score of 2. | No recommendation |
fluorouracil
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Score
2.0 |
DPYD: Normal DPD activity and "normal" risk for fluoropyrimidine toxicity | Based on genotype, there is no indication to change dose or therapy. Use label-recommended dosage and administration. | Strong | |
|
Population: No Action
|
Genotype
Phenotype
2.0 (Normal Metabolizer) |
The guideline does not provide a description of the impact of a DPYD activity score of 2 on fluorouracil. | The guideline does not provide a recommendation for fluorouracil in patients with a DPYD activity score of 2. | No recommendation | |
|
FDA Label Annotation 1 |
Genotype
DPYD:Reference/Reference
|
|
|||
|
FDA PGx Association 1 |
Genotype
DPYD:Reference/Reference
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clinical pharmacology and therapeutics. 2013. PMID:23988873
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clinical pharmacology and therapeutics. 2018. PMID:29152729
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction of DPYD and fluoropyrimidines. European journal of human genetics : EJHG. 2020. PMID:31745289
- Drugs@FDA: Drug Product Carac (fluorouracil), Bausch Health US, LLC - NDA020985/SUPPL-19, 05/26/2022.
- Drugs@FDA: Drug Product FLUOROURACIL (FLUOROURACIL), Eugia US LLC - ANDA202669/SUPPL-9, 03/21/2024.
- Drugs@FDA: Drug Product Fluorouracil (Fluorouracil), BluePoint Laboratories - ANDA210124/SUPPL-9, 03/21/2024.
- FDA Table of Pharmacogenetic Associations.
flurbiprofen
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Score
2.0 |
CYP2C9: Normal metabolism | Initiate therapy with recommended starting dose. In accordance with the prescribing information, use the lowest effective dosage for shortest duration consistent with individual patient treatment goals. | Strong | |
|
FDA Label Annotation 1 |
Genotype
CYP2C9:*1/*1
|
|
|||
|
FDA PGx Association 1 |
Genotype
CYP2C9:*1/*1
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clinical pharmacology and therapeutics. 2020. PMID:32189324
- Drugs@FDA: Drug Product FLURBIPROFEN SODIUM (flurbiprofen sodium), NDA019404, Rebel Distributors Corp.
- Drugs@FDA: Drug Product flurbiprofen (NDA018766)
- FDA Table of Pharmacogenetic Associations.
fluvastatin
In case no genotype can be determined, recommendations are based on the rs4149056 genotype alone as per guideline. The minor C allele at rs4149056 defines SLCO1B1*5.
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotypes
Activity Scores
|
|
Prescribe desired starting dose and adjust doses of fluvastatin based on disease-specific guidelines.
Other ConsiderationsThe potential for drug-drug interactions and dose limits based on renal and hepatic function and ancestry should be evaluated prior to initiating a statin. |
Strong |
Citations:
- The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 genotypes and Statin-Associated Musculoskeletal Symptoms. Clinical pharmacology and therapeutics. 2022. PMID:35152405
fosphenytoin
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Scores
|
|
No adjustments needed from typical dosing strategies. Subsequent doses should be adjusted according to therapeutic drug monitoring, response, and side effects. An HLA-B*15:02 negative test does not eliminate the risk of phenytoin-induced SJS/TEN and patients should be carefully monitored according to a usual standard. | Strong | |
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Scores
|
|
No adjustments needed from typical dosing strategies. Subsequent doses should be adjusted according to therapeutic drug monitoring, response, and side effects. An HLA-B*15:02 negative test does not eliminate the risk of phenytoin-induced SJS/TEN and patients should be carefully monitored according to a usual standard. | Strong | |
|
FDA Label Annotation 1 |
Genotypes
CYP2C9:*1/*1;HLA-B:Unknown/Unknown |
|
|||
|
Genotypes
CYP2C9:*1/*1;HLA-B:Unknown/Unknown |
|
||||
Citations:
- Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clinical pharmacology and therapeutics. 2014. PMID:25099164
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C9 and HLA-B Genotypes and Phenytoin Dosing: 2020 Update. Clinical pharmacology and therapeutics. 2021. PMID:32779747
- Drugs@FDA: Drug Product CEREBYX (Fosphenytoin Sodium), NDA020450, Pfizer Laboratories Div Pfizer Inc.
- FDA Table of Pharmacogenetic Associations.
halothane
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotypes
|
These results do not eliminate the chance that this patient is susceptible to malignant hyperthermia (MH). The genetic cause of about half of all MH survivors, with MH susceptibility confirmed by contracture test, remains unknown (PMID 28902675). | Clinical findings, family history, further genetic testing and other laboratory data should guide use of halogenated volatile anesthetics or depolarizing muscle relaxants. | Strong |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for the Use of Potent Volatile Anesthetic Agents and Succinylcholine in the Context of RYR1 or CACNA1S Genotypes. Clinical pharmacology and therapeutics. 2019. PMID:30499100
ibuprofen
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Score
2.0 |
CYP2C9: Normal metabolism | Initiate therapy with recommended starting dose. In accordance with the prescribing information, use the lowest effective dosage for shortest duration consistent with individual patient treatment goals. | Strong | |
|
FDA PGx Association 1 |
Genotype
CYP2C9:*1/*1
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clinical pharmacology and therapeutics. 2020. PMID:32189324
- FDA Table of Pharmacogenetic Associations.
imipramine
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Alternate Drug
Dosing Info
|
Genotype
Phenotypes
Activity Scores
|
|
Avoid tertiary amine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include the secondary amines nortriptyline and desipramine. For tertiary amines, consider a 50% reduction of the recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects.
Other ConsiderationsDosing recommendations only apply to higher initial doses of TCAs for treatment of conditions such as depression. Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects. Patients may receive an initial low dose of a tricyclic, which is then increased over several days to the recommended steady-state dose. The starting dose in this guideline refers to the recommended steady-state dose. |
Optional |
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
The risk of side effects is increased. The gene variation results in an increase in the plasma concentration of imipramine+desipramine. | Use 70% of the standard dose and monitor the effect and side effects or the imipramine and desipramine plasma concentrations to determine the maintenance dose, or, avoid imipramine. Antidepressants that are not or to a lesser extent metabolised by CYP2C19 include, for example, nortriptyline, fluvoxamine and mirtazapine. | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clinical pharmacology and therapeutics. 2013. PMID:23486447
- Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clinical pharmacology and therapeutics. 2017. PMID:27997040
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
irinotecan
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on irinotecan. | The guideline does not provide a recommendation for irinotecan in normal metabolizers | No recommendation | |
|
FDA Label Annotation 1 |
Genotype
UGT1A1:*1/*1
|
|
|||
|
FDA PGx Association 1 |
Genotype
UGT1A1:*1/*1
|
|
|||
Citations:
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Dutch pharmacogenetics working group (DPWG) guideline for the gene-drug interaction between UGT1A1 and irinotecan. European journal of human genetics : EJHG. 2023. PMID:36443464
- Drugs@FDA: Drug Product Camptosar (irinotecan hydrochloride), Pharmacia & Upjohn Company LLC - NDA020571/SUPPL-53, 01/27/2022.
- Drugs@FDA: Drug Product Onivyde (IRINOTECAN HYDROCHLORIDE), Ipsen Biopharmaceuticals, Inc. - NDA207793/SUPPL-16, 02/13/2024.
- FDA Table of Pharmacogenetic Associations.
isoflurane
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotypes
|
These results do not eliminate the chance that this patient is susceptible to malignant hyperthermia (MH). The genetic cause of about half of all MH survivors, with MH susceptibility confirmed by contracture test, remains unknown (PMID 28902675). | Clinical findings, family history, further genetic testing and other laboratory data should guide use of halogenated volatile anesthetics or depolarizing muscle relaxants. | Strong |
|
Population: Alternate Drug
|
Genotype
Phenotypes
|
"CONTRAINDICATIONS...with known or suspected genetic susceptibility to malignant hyperthermia...FORANE [isoflurane] can induce malignant hyperthermia in patients with known or suspected susceptibility based on genetic factors or family history, including those with certain inherited ryanodine receptor (RYR1) or dihydropyridine receptor (CACNA1S) variants." See label for more information. * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for the Use of Potent Volatile Anesthetic Agents and Succinylcholine in the Context of RYR1 or CACNA1S Genotypes. Clinical pharmacology and therapeutics. 2019. PMID:30499100
- Drugs@FDA: Drug Product FORANE (isoflurane), NDA017624, Baxter Healthcare Corporation.
ivacaftor
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: Alternate Drug
|
Genotype
Phenotype
ivacaftor non-responsive in CF patients |
CFTR: An individual diagnosed with cystic fibrosis (CF) and negative for a CFTR variant listed in the FDA-approved drug label as being responsive to ivacaftor. | Ivacaftor is not recommended | Moderate | |
|
FDA Label Annotation 1 |
Genotype
CFTR:Reference/Reference
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for ivacaftor therapy in the context of CFTR genotype. Clinical pharmacology and therapeutics. 2014. PMID:24598717
- Drugs@FDA: Drug Product Kalydeco (ivacaftor), NDA203188, Vertex Pharmaceuticals Incorporated.
lansoprazole
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Increased plasma concentration of PPI compared to CYP2C19 NMs; increased chance of efficacy and potentially toxicity | Initiate standard starting daily dose. For chronic therapy (>12 weeks) and efficacy achieved, consider 50% reduction in daily dose and monitor for continued efficacy. | Moderate |
|
Population: No Action
|
Genotype
Phenotype
Poor Metabolizer |
The higher plasma concentration of lansoprazole results in an increase in the therapeutic effectiveness, without an increase in the incidence of side effects. | NO action is needed for this gene-drug interaction. | Unspecified |
|
Affected subgroup: CYP2C19 poor metabolizers No Action
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations." * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clinical pharmacology and therapeutics. 2021. PMID:32770672
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- FDA Table of Pharmacogenetic Associations.
lornoxicam
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Score
2.0 |
CYP2C9: Normal metabolism | Initiate therapy with recommended starting dose. In accordance with the prescribing information, use the lowest effective dosage for shortest duration consistent with individual patient treatment goals. | Strong |
Citations:
- Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clinical pharmacology and therapeutics. 2020. PMID:32189324
lovastatin
In case no genotype can be determined, recommendations are based on the rs4149056 genotype alone as per guideline. The minor C allele at rs4149056 defines SLCO1B1*5.
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Function |
SLCO1B1: Typical myopathy risk and statin exposure |
Prescribe desired starting dose and adjust doses based on disease-specific guidelines.
Other ConsiderationsThe potential for drug-drug interactions and dose limits based on renal and hepatic function and ancestry should be evaluated prior to initiating a statin. |
Strong |
Citations:
- The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 genotypes and Statin-Associated Musculoskeletal Symptoms. Clinical pharmacology and therapeutics. 2022. PMID:35152405
mavacamten
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Affected subgroup: CYP2C19 intermediate or poor metabolizers Other Guidance
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations and may have higher adverse reaction risk (heart failure). Dosage is based on individual response. The dose titration and monitoring schedule accounts for differences due to CYP2C19 genetic variation, so adjustments based on CYP2C19 genotype are not necessary. Refer to FDA labeling for specific dosing recommendations and monitoring." * | Unspecified |
Citations:
meloxicam
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Score
2.0 |
CYP2C9: Normal metabolism | Initiate therapy with recommended starting dose. In accordance with the prescribing information, use the lowest effective dosage for shortest duration consistent with individual patient treatment goals. | Strong | |
|
FDA PGx Association 1 |
Genotype
CYP2C9:*1/*1
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clinical pharmacology and therapeutics. 2020. PMID:32189324
- FDA Table of Pharmacogenetic Associations.
mercaptopurine
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotypes
|
|
Start with normal starting dose (e.g., 75 mg/m2/day or 1.5 mg/kg/day) and adjust doses of mercaptopurine (and of any other myelosuppressive therapy) without any special emphasis on mercaptopurine compared to other agents. Allow at least 2 weeks to reach steady-state after each dose adjustment (PMID 20354201, 16401827, 11302950).
Other ConsiderationsNormal starting doses vary by race/ethnicity and treatment regimens. If standard dose is below normal recommended dose, dose reduction might not be recommended for intermediate metabolizers. |
Strong | |
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on mercaptopurine. | The guideline does not provide a recommendation for mercaptopurine in normal metabolizers | No recommendation | |
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on mercaptopurine. | The guideline does not provide a recommendation for mercaptopurine in normal metabolizers. | No recommendation | |
|
FDA Label Annotation 1 |
Genotypes
NUDT15:*1/*1;TPMT:*1/*1 |
|
|||
|
FDA PGx Association 1 |
Genotypes
NUDT15:*1/*1;TPMT:*1/*1 |
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clinical pharmacology and therapeutics. 2011. PMID:21270794
- Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clinical pharmacology and therapeutics. 2013. PMID:23422873
- Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clinical pharmacology and therapeutics. 2019. PMID:30447069
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Drugs@FDA: Drug Product PURINETHOL (Mercaptopurine), Quinn Pharmaceuticals - NDA009053/SUPPL-40, 12/29/2020.
- Drugs@FDA: Drug Product PURIXAN (mercaptopurine), Nova Laboratories, Ltd - NDA205919/SUPPL-4, 04/07/2020.
- FDA Table of Pharmacogenetic Associations.
methoxyflurane
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotypes
|
These results do not eliminate the chance that this patient is susceptible to malignant hyperthermia (MH). The genetic cause of about half of all MH survivors, with MH susceptibility confirmed by contracture test, remains unknown (PMID 28902675). | Clinical findings, family history, further genetic testing and other laboratory data should guide use of halogenated volatile anesthetics or depolarizing muscle relaxants. | Strong |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for the Use of Potent Volatile Anesthetic Agents and Succinylcholine in the Context of RYR1 or CACNA1S Genotypes. Clinical pharmacology and therapeutics. 2019. PMID:30499100
methylene blue
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal |
G6PD: Low risk of acute hemolytic anemia | No reason to avoid based on G6PD status | Strong | |
|
FDA Label Annotation 1 |
Genotype
G6PD:B (reference)/B (reference)
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clinical pharmacology and therapeutics. 2014. PMID:24787449
- Expanded Clinical Pharmacogenetics Implementation Consortium Guideline for Medication Use in the Context of G6PD Genotype. Clinical pharmacology and therapeutics. 2023. PMID:36049896
- Drugs@FDA: Drug Product PROVAYBLUE (methylene blue), NDA204630, American Regent, Inc.
nitrofurantoin
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal |
G6PD: Low risk of acute hemolytic anemia | No reason to avoid based on G6PD status | Strong | |
|
FDA Label Annotation 1 |
Genotype
G6PD:B (reference)/B (reference)
|
|
|||
omeprazole
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Increased plasma concentration of PPI compared to CYP2C19 NMs; increased chance of efficacy and potentially toxicity | Initiate standard starting daily dose. For chronic therapy (>12 weeks) and efficacy achieved, consider 50% reduction in daily dose and monitor for continued efficacy. | Moderate |
|
Population: No Action
|
Genotype
Phenotype
Poor Metabolizer |
The higher plasma concentration of omeprazole results in an increase in the therapeutic effectiveness, without an increase in the side effects. | NO action is required for this gene-drug interaction. | Unspecified |
|
Affected subgroup: CYP2C19 intermediate or poor metabolizers No Action
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations." * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clinical pharmacology and therapeutics. 2021. PMID:32770672
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- FDA Table of Pharmacogenetic Associations.
pantoprazole
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Increased plasma concentration of PPI compared to CYP2C19 NMs; increased chance of efficacy and potentially toxicity | Initiate standard starting daily dose. For chronic therapy (>12 weeks) and efficacy achieved, consider 50% reduction in daily dose and monitor for continued efficacy. | Moderate |
|
Population: No Action
|
Genotype
Phenotype
Poor Metabolizer |
The higher plasma concentration of pantoprazole results in an increase in the therapeutic effectiveness, without an increase in the side effects. | NO action is required for this gene-drug interaction. | Unspecified |
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
"For adult patients who are CYP2C19 poor metabolizers, no dosage adjustment is needed...For known pediatric poor metabolizers, a dose reduction should be considered." See label for more information. * | Unspecified | |
|
Pediatrics; Affected subgroup: CYP2C19 intermediate or poor metabolizers Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations. Consider dosage reduction in children who are poor metabolizers. No dosage adjustment is needed for adult patients who are intermediate or poor metabolizers." * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clinical pharmacology and therapeutics. 2021. PMID:32770672
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Drugs@FDA: Drug Product Protonix Delayed-Release (PANTOPRAZOLE SODIUM), NDA020987, Avera McKennan Hospital.
- FDA Table of Pharmacogenetic Associations.
pegloticase
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal |
G6PD: Low risk of acute hemolytic anemia | No reason to avoid based on G6PD status | Strong | |
|
FDA Label Annotation 1 |
Genotype
G6PD:B (reference)/B (reference)
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clinical pharmacology and therapeutics. 2014. PMID:24787449
- Expanded Clinical Pharmacogenetics Implementation Consortium Guideline for Medication Use in the Context of G6PD Genotype. Clinical pharmacology and therapeutics. 2023. PMID:36049896
- Drugs@FDA: Drug Product Krystexxa (pegloticase), BLA125293, Horizon Pharma Inc.
phenprocoumon
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
-1639 GG |
The guideline does not provide a description of the impact of the VKORC1 rs9923231 CC genotype (-1639 GG genotype) on phenprocoumon. | The guideline does not provide a recommendation for phenprocoumon in patients with the VKORC1 rs9923231 CC genotype (-1639 GG genotype). | No recommendation |
Citations:
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
phenytoin
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Scores
|
|
No adjustments needed from typical dosing strategies. Subsequent doses should be adjusted according to therapeutic drug monitoring, response, and side effects. An HLA-B*15:02 negative test does not eliminate the risk of phenytoin-induced SJS/TEN and patients should be carefully monitored according to a usual standard. | Strong | |
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Scores
|
|
No adjustments needed from typical dosing strategies. Subsequent doses should be adjusted according to therapeutic drug monitoring, response, and side effects. An HLA-B*15:02 negative test does not eliminate the risk of phenytoin-induced SJS/TEN and patients should be carefully monitored according to a usual standard. | Strong | |
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on phenytoin. | The guideline does not provide a recommendation for phenytoin in normal metabolizers. | No recommendation | |
|
FDA Label Annotation 1 |
Genotypes
CYP2C9:*1/*1;HLA-B:Unknown/Unknown |
|
|||
|
Genotypes
CYP2C9:*1/*1;HLA-B:Unknown/Unknown |
|
||||
Citations:
- Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clinical pharmacology and therapeutics. 2014. PMID:25099164
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C9 and HLA-B Genotypes and Phenytoin Dosing: 2020 Update. Clinical pharmacology and therapeutics. 2021. PMID:32779747
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction of CYP2C9, HLA-A and HLA-B with anti-epileptic drugs. European journal of human genetics : EJHG. 2024. PMID:38570725
- Drugs@FDA Drug Product: DILANTIN (phenytoin), NDA008762, Upjohn.
- FDA Table of Pharmacogenetic Associations.
piroxicam
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Score
2.0 |
CYP2C9: Normal metabolism | Initiate therapy with recommended starting dose. In accordance with the prescribing information, use the lowest effective dosage for shortest duration consistent with individual patient treatment goals. | Strong | |
|
FDA Label Annotation 1 |
Genotype
CYP2C9:*1/*1
|
|
|||
|
FDA PGx Association 1 |
Genotype
CYP2C9:*1/*1
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clinical pharmacology and therapeutics. 2020. PMID:32189324
- Drugs@FDA: Drug Product Feldene (piroxicam), NDA018147, Pfizer Laboratories Div Pfizer Inc.
- FDA Table of Pharmacogenetic Associations.
pitavastatin
In case no genotype can be determined, recommendations are based on the rs4149056 genotype alone as per guideline. The minor C allele at rs4149056 defines SLCO1B1*5.
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Function |
SLCO1B1: Typical myopathy risk and statin exposure |
Prescribe desired starting dose and adjust doses based on disease-specific guidelines.
Other ConsiderationsThe potential for drug-drug interactions and dose limits based on renal and hepatic function and ancestry should be evaluated prior to initiating a statin. |
Strong |
Citations:
- The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 genotypes and Statin-Associated Musculoskeletal Symptoms. Clinical pharmacology and therapeutics. 2022. PMID:35152405
pravastatin
In case no genotype can be determined, recommendations are based on the rs4149056 genotype alone as per guideline. The minor C allele at rs4149056 defines SLCO1B1*5.
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Function |
SLCO1B1: Typical myopathy risk and statin exposure |
Prescribe desired starting dose and adjust doses based on disease-specific guidelines.
Other ConsiderationsThe potential for drug-drug interactions and dose limits based on renal and hepatic function and ancestry should be evaluated prior to initiating a statin. |
Strong |
Citations:
- The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 genotypes and Statin-Associated Musculoskeletal Symptoms. Clinical pharmacology and therapeutics. 2022. PMID:35152405
primaquine
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal |
G6PD: Low risk of acute hemolytic anemia | No reason to avoid based on G6PD status | Strong | |
|
FDA Label Annotation 1 |
Genotype
G6PD:B (reference)/B (reference)
|
|
|||
Citations:
- Expanded Clinical Pharmacogenetics Implementation Consortium Guideline for Medication Use in the Context of G6PD Genotype. Clinical pharmacology and therapeutics. 2023. PMID:36049896
- Drugs@FDA: Drug Product Primaquine Phosphate (Primaquine Phosphate), NDA008316, PD-Rx Pharmaceuticals, Inc.
quetiapine
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on quetiapine. | The guideline does not provide a recommendation for quetiapine in normal metabolizers. | No recommendation |
Citations:
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2D6, CYP3A4 and CYP1A2 and antipsychotics. European journal of human genetics : EJHG. 2024. PMID:37002327
rabeprazole
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Affected subgroup: CYP2C19 poor metabolizers No Action
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations." * | Unspecified |
Citations:
rasburicase
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal |
G6PD: Low risk of acute hemolytic anemia | No reason to avoid based on G6PD status | Strong | |
|
FDA Label Annotation 1 |
Genotype
G6PD:B (reference)/B (reference)
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clinical pharmacology and therapeutics. 2014. PMID:24787449
- Expanded Clinical Pharmacogenetics Implementation Consortium Guideline for Medication Use in the Context of G6PD Genotype. Clinical pharmacology and therapeutics. 2023. PMID:36049896
- Drugs@FDA: Drug Product Elitek (rasburicase), BLA103946, sanofi-aventis U.S. LLC.
rosuvastatin
In case no genotype can be determined, recommendations are based on the rs4149056 genotype alone as per guideline. The minor C allele at rs4149056 defines SLCO1B1*5.
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotypes
|
|
Prescribe desired starting dose and adjust doses of rosuvastatin based on disease-specific and specific population guidelines.
Other ConsiderationsThe potential for drug-drug interactions and dose limits based on renal and hepatic function and Asian ancestry should be evaluated prior to initiating rosuvastatin. |
Strong | |
|
Population: No Action
|
Genotype
Phenotype
Normal Function |
The guideline does not provide a recommendation for rosuvastatin in patients with the SLCO1B1 521 TT genotype | No recommendation | ||
|
FDA PGx Association 1 |
Genotype
SLCO1B1:*1/*1
|
|
|||
Citations:
- The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 genotypes and Statin-Associated Musculoskeletal Symptoms. Clinical pharmacology and therapeutics. 2022. PMID:35152405
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between SLCO1B1 and statins and CYP2C9 and sulfonylureas. European journal of human genetics : EJHG. 2024. PMID:39676086
- FDA Table of Pharmacogenetic Associations.
sertraline
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Alternate Drug
Dosing Info
|
Genotype
Phenotypes
|
|
Consider a lower starting dose, slower titration schedule and 50% reduction of standard maintenance dose as compared to CYP2C19 normal metabolizers or select a clinically appropriate alternative antidepressant not predominantly metabolized by CYP2C19. | Moderate |
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
The risk of side effects is increased. The gene variation leads to increased plasma concentrations of sertraline. | Do not give doses exceeding 75 mg/day. Guide the dose by response and side effects and/or sertraline plasma concentration. | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clinical pharmacology and therapeutics. 2015. PMID:25974703
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clinical pharmacology and therapeutics. 2023. PMID:37032427
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. European journal of human genetics : EJHG. 2022. PMID:34782755
sevoflurane
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotypes
|
These results do not eliminate the chance that this patient is susceptible to malignant hyperthermia (MH). The genetic cause of about half of all MH survivors, with MH susceptibility confirmed by contracture test, remains unknown (PMID 28902675). | Clinical findings, family history, further genetic testing and other laboratory data should guide use of halogenated volatile anesthetics or depolarizing muscle relaxants. | Strong |
|
Population: Alternate Drug
|
Genotype
Phenotypes
|
"CONTRAINDICATIONS: Known or suspected genetic susceptibility to malignant hyperthermia...ULTANE [sevoflurane] can induce malignant hyperthermia in patients with known or suspected susceptibility based on genetic factors or family history, including those with certain inherited ryanodine receptor (RYR1) or dihydropyridine receptor (CACNA1S) variants." See label for more information. * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for the Use of Potent Volatile Anesthetic Agents and Succinylcholine in the Context of RYR1 or CACNA1S Genotypes. Clinical pharmacology and therapeutics. 2019. PMID:30499100
- Drugs@FDA: Drug Product Ultane (Sevoflurane), NDA020478, AbbVie Inc.
simvastatin
In case no genotype can be determined, recommendations are based on the rs4149056 genotype alone as per guideline. The minor C allele at rs4149056 defines SLCO1B1*5.
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Function |
SLCO1B1: Typical myopathy risk and statin exposure |
Prescribe desired starting dose and adjust doses based on disease-specific guidelines.
Other ConsiderationsThe potential for drug-drug interactions and dose limits based on renal and hepatic function and ancestry should be evaluated prior to initiating a statin. |
Strong | |
|
Population: No Action
|
Genotype
Phenotype
Normal Function |
The guideline does not provide a description of the impact of the SLCO1B1 521 TT genotype on simvastatin. | The guideline does not provide a recommendation for atorvastatin in patients with the SLCO1B1 521 TT genotype. | No recommendation | |
|
FDA PGx Association 1 |
Genotype
SLCO1B1:*1/*1
|
|
|||
Citations:
- The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clinical pharmacology and therapeutics. 2012. PMID:22617227
- The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clinical pharmacology and therapeutics. 2014. PMID:24918167
- The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 genotypes and Statin-Associated Musculoskeletal Symptoms. Clinical pharmacology and therapeutics. 2022. PMID:35152405
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between SLCO1B1 and statins and CYP2C9 and sulfonylureas. European journal of human genetics : EJHG. 2024. PMID:39676086
- FDA Table of Pharmacogenetic Associations.
siponimod
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on siponimod. | The guideline does not provide a recommendation for siponimod in normal metabolizers. | No recommendation | |
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Score
2.0 |
The MAYZENT (siponimod) label states: "Recommended Dosage in Patients With CYP2C9 Genotypes *1/*1, *1/*2, or *2/*2" "Initiate MAYZENT with a 5-day titration, as shown in Table 1... A starter pack should be used for patients who will be titrated to the 2-mg maintenance dosage" "After treatment titration (see Treatment Initiation), the recommended maintenance dosage of MAYZENT is 2 mg taken orally once daily starting on Day 6." See label for more information. * | Unspecified | ||
|
FDA PGx Association 1 |
Genotype
CYP2C9:*1/*1
|
|
|||
succinylcholine
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Other Guidance
|
Genotype
Phenotypes
|
These results do not eliminate the chance that this patient is susceptible to malignant hyperthermia (MH). The genetic cause of about half of all MH survivors, with MH susceptibility confirmed by contracture test, remains unknown (PMID 28902675). | Clinical findings, family history, further genetic testing and other laboratory data should guide use of halogenated volatile anesthetics or depolarizing muscle relaxants. | Strong |
|
Population: Alternate Drug
Other Guidance
|
Genotype
Phenotypes
|
"ANECTINE [succinylcholine] is contraindicated in patients with...[k]nown or suspected genetic susceptibility to malignant hyperthermia...Anectine [succinylcholine] can induce malignant hyperthermia in patients with known or suspected susceptibility based on genetic factors or family history, including those with certain inherited ryanodine receptor (RYR1) or dihydropyridine receptor (CACNA1S) variants...Succinylcholine is metabolized by plasma cholinesterase and should be used with caution, if at all, in patients known to be or suspected of being homozygous for the atypical plasma cholinesterase gene [BCHE] ." See label for more information. * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for the Use of Potent Volatile Anesthetic Agents and Succinylcholine in the Context of RYR1 or CACNA1S Genotypes. Clinical pharmacology and therapeutics. 2019. PMID:30499100
- Drugs@FDA: Drug Product Anectine (Succinylcholine Chloride), NDA008453, Sandoz Inc.
tacrolimus
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Dosing Info
|
Genotype
Phenotype
Normal Metabolizer |
CYP3A5: Lower dose-adjusted trough concentrations of tacrolimus and decreased chance of achieving target tacrolimus concentrations. |
Increase starting dose 1.5 to 2 times recommended starting dose. Total starting dose should not exceed 0.3 mg/kg/day. Use therapeutic drug monitoring to guide dose adjustments.
Other ConsiderationsThis recommendation includes the use of tacrolimus in kidney, heart, lung and hematopoietic stem cell transplant patients, and liver transplant patients where the donor and recipient genotypes are identical. Further dose adjustments or selection of alternative therapy may be necessary due to other clinical factors (e.g., medication interactions, or hepatic function). Typically with other CYP enzymes, a normal metabolizer would be classified as having normal metabolism, and therefore, the drug dose would not change based on the patient’s genotype. However, in the case of CYP3A5 and tacrolimus, a CYP3A5 expresser (i.e., CYP3A5 normal metabolizer or intermediate metabolizer) would require a higher recommended starting dose, and the CYP3A5 non-expresser (i.e., poor metabolizer) would require the standard recommended starting dose. |
Strong |
|
Population: Dosing Info
|
Genotype
Phenotype
Normal Metabolizer |
An increase of the initial dose can result in an increased chance of reaching a tacrolimus concentration within the target range before the start of therapeutic drug monitoring. However, there is no direct evidence that this results in improved clinical results. The genetic variation results in an increased conversion of tacrolimus to inactive metabolites and therefore a higher required dose. | LIVER TRANSPLANTATION In addition to the patient’s genotype, the metabolism of tacrolimus is also determined by the genotype of the transplanted liver. LIVER is also of the genotype HOMOZYGOUS EXPRESSOR: Use 2.5 times the normal initial dose. Adjustment of the dose should then be based on therapeutic drug monitoring. LIVER has a DIFFERENT genotype: There is insufficient evidence in the literature to support a dose recommendation. OTHER TRANSPLANTATION Use 2.5 times the initial dose that would yield the desired result in non-expressers. Adjustment of the dose should then be based on therapeutic drug monitoring. For example: One Dutch study found a median trough concentration for tacrolimus after three days of 9.4 ng/mL at an initial dose of 0.15 mg/kg twice daily for 5 homozygous kidney transplant patients. Their target value was 10 - 15 ng/mL. | Unspecified |
|
Affected subgroup: CYP3A5 intermediate or normal metabolizers Dosing Info
|
Genotype
Phenotype
Normal Metabolizer |
"Results in lower systemic concentrations, lower probability of achieving target concentrations and may result in higher rejection risk. Measure drug concentrations and adjust dosage based on trough whole blood tacrolimus concentrations." * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clinical pharmacology and therapeutics. 2015. PMID:25801146
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- FDA Table of Pharmacogenetic Associations.
tafenoquine
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal |
G6PD: Low risk of acute hemolytic anemia |
No reason to avoid based on G6PD status
Other ConsiderationsTafenoquine’s safety has been established for a G6PD enzyme activity ≥70% of normal. (Inclusion criteria for clinical trials involving tafenoquine included G6PD activity ≥70%.) |
Strong | |
|
FDA Label Annotation 1 |
Genotype
G6PD:B (reference)/B (reference)
|
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clinical pharmacology and therapeutics. 2014. PMID:24787449
- Expanded Clinical Pharmacogenetics Implementation Consortium Guideline for Medication Use in the Context of G6PD Genotype. Clinical pharmacology and therapeutics. 2023. PMID:36049896
- Drugs@FDA: Drug Product Arakoda (Tafenoquine), NDA210607, 60 Degrees Pharmaceuticals, LLC.
tegafur
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
2.0 (Normal Metabolizer) |
The guideline does not provide a description of the impact of a DPYD activity score of 2 on tegafur. | The guideline does not provide a recommendation for tegafur in patients with a DPYD activity score of 2. | No recommendation |
Citations:
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction of DPYD and fluoropyrimidines. European journal of human genetics : EJHG. 2020. PMID:31745289
tenoxicam
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer Activity Score
2.0 |
CYP2C9: Normal metabolism | Initiate therapy with recommended starting dose. In accordance with the prescribing information, use the lowest effective dosage for shortest duration consistent with individual patient treatment goals. | Strong |
Citations:
- Clinical Pharmacogenetics Implementation Consortium Guideline (CPIC) for CYP2C9 and Nonsteroidal Anti-Inflammatory Drugs. Clinical pharmacology and therapeutics. 2020. PMID:32189324
thioguanine
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotypes
|
|
Start with normal starting dose (e.g., 40-60 mg/m2/day) and adjust doses of thioguanine and of other myelosuppressive therapy without any special emphasis on thioguanine. Allow 2 weeks to reach steady-state after each dose adjustment (PMID 20354201, 11037857).
Other ConsiderationsNormal starting doses vary by race/ethnicity and treatment regimens. If standard dose is below normal recommended dose, dose reduction might not be recommended for intermediate metabolizers. |
Strong | |
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on thioguanine. | The guideline does not provide a recommendation for thioguanine in normal metabolizers | No recommendation | |
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on thioguanine. | The guideline does not provide a recommendation for thioguanine in normal metabolizers. | No recommendation | |
|
FDA Label Annotation 1 |
Genotypes
NUDT15:*1/*1;TPMT:*1/*1 |
|
|||
|
FDA PGx Association 1 |
Genotypes
NUDT15:*1/*1;TPMT:*1/*1 |
|
|||
Citations:
- Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clinical pharmacology and therapeutics. 2011. PMID:21270794
- Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clinical pharmacology and therapeutics. 2013. PMID:23422873
- Clinical Pharmacogenetics Implementation Consortium Guideline for Thiopurine Dosing Based on TPMT and NUDT15 Genotypes: 2018 Update. Clinical pharmacology and therapeutics. 2019. PMID:30447069
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- Drugs@FDA: Drug Product TABLOID (thioguanine), NDA012429, Aspen Global Inc.
- FDA Table of Pharmacogenetic Associations.
toluidine blue
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: No Action
|
Genotype
Phenotype
Normal |
G6PD: Low risk of acute hemolytic anemia |
No reason to avoid based on G6PD status
Other ConsiderationsToluidine blue classification strength is based on extrapolation from methylene blue data |
Strong |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for rasburicase therapy in the context of G6PD deficiency genotype. Clinical pharmacology and therapeutics. 2014. PMID:24787449
- Expanded Clinical Pharmacogenetics Implementation Consortium Guideline for Medication Use in the Context of G6PD Genotype. Clinical pharmacology and therapeutics. 2023. PMID:36049896
trimipramine
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Alternate Drug
Dosing Info
|
Genotype
Phenotypes
Activity Scores
|
|
Avoid tertiary amine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include the secondary amines nortriptyline and desipramine. For tertiary amines, consider a 50% reduction of the recommended starting dose. Utilize therapeutic drug monitoring to guide dose adjustments. Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects.
Other ConsiderationsDosing recommendations only apply to higher initial doses of TCAs for treatment of conditions such as depression. Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects. Patients may receive an initial low dose of a tricyclic, which is then increased over several days to the recommended steady-state dose. The starting dose in this guideline refers to the recommended steady-state dose. |
Optional |
Citations:
- Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clinical pharmacology and therapeutics. 2013. PMID:23486447
- Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clinical pharmacology and therapeutics. 2017. PMID:27997040
voriconazole
| Source | Genes | Implications | Recommendation | Classification |
|---|---|---|---|---|
|
Population: Alternate Drug
Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Higher dose-adjusted trough concentrations of voriconazole and may increase probability of adverse events |
Choose an alternative agent that is not dependent on CYP2C19 metabolism as primary therapy in lieu of voriconazole. Such agents include isavuconazole, liposomal amphotericin B, and posaconazole. In the event that voriconazole is considered to be the most appropriate agent, based on clinical advice, for a patient with poor metabolizer genotype, voriconazole should be administered at a preferably lower than standard dosage with careful therapeutic drug monitoring.
Other ConsiderationsFurther dose adjustments or selection of alternative therapy may be necessary due to other clinical factors, such as drug interactions, hepatic function, renal function, species, site of infection, therapeutic drug monitoring, and comorbidities. |
Moderate |
|
Population: Alternate Drug
Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
CYP2C19: Higher dose-adjusted trough concentrations of voriconazole and may increase probability of adverse events |
Choose an alternative agent that is not dependent on CYP2C19 metabolism as primary therapy in lieu of voriconazole. Such agents include liposomal amphotericin B and posaconazole. In the event that voriconazole is considered to be the most appropriate agent, based on clinical advice, for a patient with poor metabolizer genotype, voriconazole should be administered at a preferably lower than standard dosage with careful therapeutic drug monitoring.
Other ConsiderationsFurther dose adjustments or selection of alternative therapy may be necessary due to other clinical factors, such as drug interactions, hepatic function, renal function, species, site of infection, therapeutic drug monitoring, and comorbidities. Recommendation based upon data extrapolated from adults. |
Moderate |
|
Population: Dosing Info
|
Genotype
Phenotype
Poor Metabolizer |
The gene variation can reduce the conversion of voriconazole and consequently increase the plasma concentration. This could result in improved efficacy or an increase in the risk of side effects. Initially, the risk of side effects is of particular interest. | Use 50% of the standard dose and monitor the plasma concentration. | Unspecified |
|
Affected subgroup: CYP2C19 intermediate or poor metabolizers Other Guidance
|
Genotype
Phenotype
Poor Metabolizer |
"Results in higher systemic concentrations and may result in higher adverse reaction risk." * | Unspecified |
Citations:
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy. Clinical pharmacology and therapeutics. 2017. PMID:27981572
- Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011. PMID:21412232
- FDA Table of Pharmacogenetic Associations.
warfarin
| Source | Genes | Implications | Recommendation | Classification | |
|---|---|---|---|---|---|
|
Population: |
Genotype
|
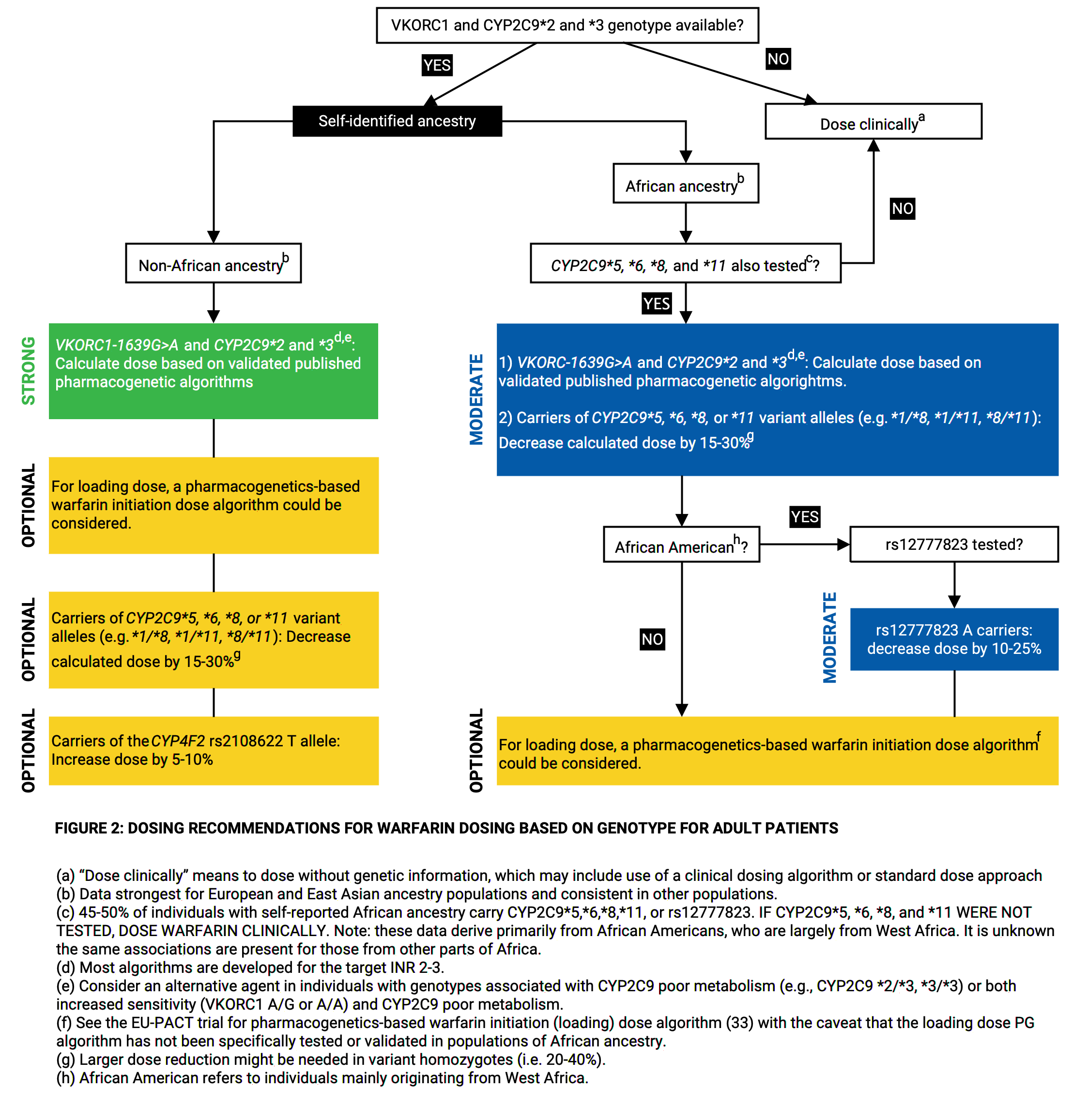
|
|||
|
Population: No Action
|
Genotype
Phenotype
Normal Metabolizer |
The guideline does not provide a description of the impact of a normal metabolizer phenotype on warfarin. | The guideline does not provide a recommendation for warfarin in normal metabolizers. | No recommendation | |
|
Population: No Action
|
Genotype
Phenotype
-1639 GG |
The guideline does not provide a description of the impact of the VKORC1 rs9923231 CC genotype (-1639 GG genotype) on warfarin. | The guideline does not provide a recommendation for warfarin in patients with the VKORC1 rs9923231 CC genotype (-1639 GG genotype). | No recommendation | |
|
Population: Dosing Info
|
Genotype
Phenotype
Normal Metabolizer Activity Scores
|
Table 1 in the drug label provides ranges of expected maintenance daily doses of warfarin (COUMADIN) based on CYP2C9*2, CYP2C9*3 and VKORC1−1639G>A (rs9923231) genotypes. For CYP2C9*1/*1, VKORC1 GG (rs9923231CC), the range of expected maintenance daily dose is 5-7 mg. * | Unspecified | ||
|
Genotypes
CYP2C9:*1/*1;VKORC1:rs9923231 reference (C)/rs9923231 reference (C) |
|
||||
Citations:
- Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clinical pharmacology and therapeutics. 2011. PMID:21900891
- Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Pharmacogenetics-Guided Warfarin Dosing: 2017 Update. Clinical pharmacology and therapeutics. 2017. PMID:28198005
- Drugs@FDA: Drug Product COUMADIN (warfarin sodium), NDA009218, Bristol-Myers Squibb Pharma Company.
- FDA Table of Pharmacogenetic Associations.
Drugs With No Guidance
The following drugs are known to be associated with genes in this report but have no guidance for the specific genotypes in this report. For more information, see the "Prescribing Info" page on PharmGKB.
- Ascorbic acid (vitamin C), combinations
- Ascorbic acid (vitamin C), plain
- articaine / epinephrine
- avatrombopag
- belinostat
- bupivacaine
- chloroquine
- chlorpropamide
- dabrafenib
- deuruxolitinib
- dolutegravir
- dronabinol
- elagolix
- erdafitinib
- flutamide
- glimepiride
- glipizide
- glyburide
- hydroxychloroquine
- lesinurad
- lidocaine / prilocaine
- lidocaine and tetracaine
- mepivacaine
- moviprep
- nalidixic acid
- nateglinide
- nilotinib
- oxymetazoline and tetracaine
- pazopanib
- peginterferon alfa-2a
- peginterferon alfa-2b
- raltegravir
- ribavirin
- ropivacaine
- sacituzumab govitecan
- sodium ascorbate
- sodium nitrite
- sulfasalazine
- tolazamide
- tolbutamide
Section III: Allele Matching Details
- ABCG2 allele match data
- CACNA1S allele match data
- CFTR allele match data
- CYP2B6 allele match data
- CYP2C19 allele match data
- CYP2C9 allele match data
- CYP3A4 allele match data
- CYP3A5 allele match data
- CYP4F2 allele match data
- DPYD allele match data
- G6PD allele match data
- IFNL3/4 allele match data
- NUDT15 allele match data
- RYR1 allele match data
- SLCO1B1 allele match data
- TPMT allele match data
- UGT1A1 allele match data
- VKORC1 allele match data
No data provided for CYP2D6.
ABCG2 allele match data
| Variant Matched: | rs2231142 reference (G)/rs2231142 reference (G) |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr4:88131171 | rs2231142 | G/G | G |
|
CACNA1S allele match data
| Variant Matched: | Reference/Reference |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr1:201060815 | rs1800559 | C/C | C |
|
|
| chr1:201091993 | rs772226819 | G/G | G |
|
CFTR allele match data
| Variant Matched: | Reference/Reference |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr7:117509035 | rs397508256 | G/G | G |
|
|
| chr7:117509069 | rs368505753 | C/C | C |
|
|
| chr7:117509089 | rs115545701 | C/C | C |
|
|
| chr7:117530953 | rs113993958 | G/G | G |
|
|
| chr7:117530955 | rs397508537 | C/C | C |
|
|
| chr7:117530974 | rs77834169 | C/C | C |
|
|
| chr7:117530975 | rs78655421 | G/G | G |
|
|
| chr7:117534318 | rs80282562 | G/G | G |
|
|
| chr7:117534363 | rs397508759 | G/G | G |
|
|
| chr7:117534368 | rs397508761 | A/A | A |
|
|
| chr7:117535285 | rs121908752 | T/T | T |
|
|
| chr7:117540270 | rs77932196 | G/G | G |
|
|
| chr7:117540285 | rs121908753 | G/G | G |
|
|
| chr7:117548795 | rs74551128 | C/C | C |
|
|
| chr7:117587799 | rs121908757 | A/A | A |
|
|
| chr7:117587800 | rs121908755 | G/G | G |
|
|
| chr7:117587801 | rs121909005 | T/T | T |
|
|
| chr7:117587805 | rs121909013 | G/G | G |
|
|
| chr7:117587806 | rs75527207 | G/G | G |
|
|
| chr7:117590409 | rs397508288 | A/A | A |
|
|
| chr7:117594930 | rs397508387 | G/G | G |
|
|
| chr7:117602868 | rs80224560 | G/G | G |
|
|
| chr7:117603708 | rs397508442 | C/C | C |
|
|
| chr7:117606695 | rs141033578 | C/C | C |
|
|
| chr7:117611555 | rs76151804 | A/A | A |
|
|
| chr7:117611595 | rs150212784 | T/T | T |
|
|
| chr7:117611620 | rs397508513 | A/A | A |
|
|
| chr7:117611640 | rs121909020 | G/G | G |
|
|
| chr7:117611646 | rs200321110 | G/G | G |
|
|
| chr7:117611649 | rs202179988 | C/C | C |
|
|
| chr7:117611650 | rs78769542 | G/G | G |
|
|
| chr7:117611663 | rs186045772 | T/T | T |
|
|
| chr7:117614699 | rs75541969 | G/G | G |
|
|
| chr7:117639961 | rs75039782 | C/C | C |
|
|
| chr7:117642451 | rs267606723 | G/G | G |
|
|
| chr7:117642472 | rs74503330 | G/G | G |
|
|
| chr7:117642483 | rs121909041 | T/T | T |
|
|
| chr7:117642528 | rs11971167 | G/G | G |
|
|
| chr7:117664770 | rs193922525 | G/G | G |
|
CYP2B6 allele match data
| Allele Matched: | *1/*6 |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr19:40991224 | rs34223104 | T/T | T |
|
|
| chr19:40991367 | rs34883432 | A/A | A |
|
|
| chr19:40991369 | rs8192709 | C/C | C |
|
|
| chr19:40991381 | rs33973337 | A/A | A |
|
|
| chr19:40991388 | rs33980385 | A/A | A |
|
|
| chr19:40991390 | rs33926104 | C/C | C |
|
|
| chr19:40991391 | rs34284776 | G/G | G |
|
|
| chr19:40991441 | rs35303484 | A/A | A |
|
|
| chr19:41004015 | rs281864907 | T/T | T |
|
|
| chr19:41004125 | rs36060847 | G/G | G |
|
|
| chr19:41004133 | rs148009906 | G/G | G |
|
|
| chr19:41004158 | rs186335453 | G/G | G |
|
|
| chr19:41004303 | rs139801276 | T/T | T |
|
|
| chr19:41004377 | rs12721655 | A/A | A |
|
|
| chr19:41004380 | rs535039125 | C/C | C |
|
|
| chr19:41004381 | rs35773040 | G/G | G |
|
|
| chr19:41004406 | rs145884402 | G/G | G |
|
|
| chr19:41004435 | rs141666881 | G/G | G |
|
|
| chr19:41006919 | rs3826711 | C/C | C |
|
|
| chr19:41006923 | rs36056539 | C/C | C |
|
|
| chr19:41006936 | rs3745274 | G/T | G |
|
|
| chr19:41006967 | rs58871670 | G/G | G |
|
|
| chr19:41006968 | rs373489637 | T/T | T |
|
|
| chr19:41007013 | rs36079186 | T/T | T |
|
|
| chr19:41009313 | A/A | A |
|
||
| chr19:41009350 | rs45482602 | C/C | C |
|
|
| chr19:41009358 | rs2279343 | A/G | A |
|
|
| chr19:41010006 | rs139029625 | G/G | G |
|
|
| chr19:41010088 | rs34698757 | C/C | C |
|
|
| chr19:41010108 | rs193922917 | C/C | C |
|
|
| chr19:41012316 | rs28399499 | T/T | T |
|
|
| chr19:41012339 | rs34826503 | C/C | C |
|
|
| chr19:41012393 | rs754621576 | T/T | T |
|
|
| chr19:41012394 | rs780991919 | A/A | A |
|
|
| chr19:41012465 | rs34097093 | C/C | C |
|
|
| chr19:41012466 | rs200458614 | G/G | G |
|
|
| chr19:41012471 | rs201500445 | T/T | T |
|
|
| chr19:41012478 | rs200238771 | T/T | T |
|
|
| chr19:41012693 | rs35979566 | T/T | T |
|
|
| chr19:41012740 | rs193922918 | G/G | G |
|
|
| chr19:41012803 | rs35010098 | C/C | C |
|
|
| chr19:41016652 | rs764288403 | G/G | G |
|
|
| chr19:41016679 | rs374099483 | G/G | G |
|
|
| chr19:41016726 | rs3211369 | A/A | A |
|
|
| chr19:41016741 | rs117872433 | G/G | G |
|
|
| chr19:41016778 | rs564083989 | G/G | G |
|
|
| chr19:41016805 | A/A | A |
|
||
| chr19:41016810 | rs3211371 | C/C | C |
|
CYP2C19 allele match data
| Allele Matched: | *2/*2 |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr10:94761900 | rs12248560 | C/C | C |
|
|
| chr10:94762706 | rs28399504 | A/A | A |
|
|
| chr10:94762712 | rs367543002 | C/C | C |
|
|
| chr10:94762715 | rs367543003 | T/T | T |
|
|
| chr10:94762755 | rs55752064 | T/T | T |
|
|
| chr10:94762760 | rs17882687 | A/A | A |
|
|
| chr10:94762788 | rs1564656981 | A/A | A |
|
|
| chr10:94762856 | rs1564657013 | A/A | A |
|
|
| chr10:94775106 | rs145328984 | C/C | C |
|
|
| chr10:94775121 | rs1564660997 | C/C | C |
|
|
| chr10:94775160 | rs118203756 | G/G | G |
|
|
| chr10:94775185 | rs1288601658 | A/A | A |
|
|
| chr10:94775367 | rs12769205 | G/G | A |
|
|
| chr10:94775416 | rs41291556 | T/T | T |
|
|
| chr10:94775423 | rs17885179 | A/A | A |
|
|
| chr10:94775453 | rs72552267 | G/G | G |
|
|
| chr10:94775489 | rs17884712 | G/G | G |
|
|
| chr10:94775507 | rs58973490 | G/G | G |
|
|
| chr10:94780574 | rs140278421 | G/G | G |
|
|
| chr10:94780579 | rs370803989 | G/G | G |
|
|
| chr10:94780653 | rs4986893 | G/G | G |
|
|
| chr10:94781858 | rs6413438 | C/C | C |
|
|
| chr10:94781859 | rs4244285 | A/A | G |
|
|
| chr10:94781944 | rs375781227 | G/G | G |
|
|
| chr10:94781999 | rs72558186 | T/T | T |
|
|
| chr10:94842861 | rs138142612 | G/G | G |
|
|
| chr10:94842866 | rs3758581 | G/G | A |
|
|
| chr10:94842879 | rs118203757 | G/G | G |
|
|
| chr10:94842995 | rs113934938 | G/G | G |
|
|
| chr10:94849995 | rs17879685 | C/C | C |
|
|
| chr10:94852738 | rs56337013 | C/C | C |
|
|
| chr10:94852765 | rs192154563 | C/C | C |
|
|
| chr10:94852785 | rs118203759 | C/C | C |
|
|
| chr10:94852914 | rs55640102 | A/A | A |
|
CYP2C9 allele match data
| Allele Matched: | *1/*1 |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr10:94938683 | rs114071557 | A/A | A |
|
|
| chr10:94938719 | T/T | T |
|
||
| chr10:94938737 | rs67807361 | C/C | C |
|
|
| chr10:94938771 | rs142240658 | C/C | C |
|
|
| chr10:94938788 | C/C | C |
|
||
| chr10:94938800 | rs1364419386 | G/G | G |
|
|
| chr10:94938803 | rs2031308986 | A/A | A |
|
|
| chr10:94938828 | rs564813580 | A/A | A |
|
|
| chr10:94941897 | rs371055887 | G/G | G |
|
|
| chr10:94941915 | G/G | G |
|
||
| chr10:94941958 | rs72558187 | T/T | T |
|
|
| chr10:94941975 | G/G | G |
|
||
| chr10:94941976 | G/G | G |
|
||
| chr10:94941982 | rs762239445 | G/G | G |
|
|
| chr10:94942018 | T/T | T |
|
||
| chr10:94942205 | rs1304490498 | CAATGGAAA GA/ CAATGGAAA GA |
CAATGGAAA GA |
|
|
| chr10:94942216 | rs774607211 | A/A | A |
|
|
| chr10:94942230 | rs767576260 | C/C | C |
|
|
| chr10:94942231 | rs12414460 | G/G | G |
|
|
| chr10:94942233 | rs375805362 | C/C | C |
|
|
| chr10:94942234 | rs72558189 | G/G | G |
|
|
| chr10:94942243 | rs1375956433 | T/T | T |
|
|
| chr10:94942249 | rs200965026 | C/C | C |
|
|
| chr10:94942254 | rs199523631 | C/C | C |
|
|
| chr10:94942255 | rs200183364 | G/G | G |
|
|
| chr10:94942290 | rs1799853 | C/C | C |
|
|
| chr10:94942291 | rs141489852 | G/G | G |
|
|
| chr10:94942305 | rs754487195 | G/G | G |
|
|
| chr10:94942306 | rs1289704600 | C/C | C |
|
|
| chr10:94942308 | rs17847037 | C/C | C |
|
|
| chr10:94942309 | rs7900194 | G/G | G |
|
|
| chr10:94947782 | rs72558190 | C/C | C |
|
|
| chr10:94947785 | rs774550549 | C/C | C |
|
|
| chr10:94947869 | A/A | A |
|
||
| chr10:94947907 | A/A | A |
|
||
| chr10:94947917 | rs1326630788 | T/T | T |
|
|
| chr10:94947938 | rs2031531005 | A/A | A |
|
|
| chr10:94947939 | rs370100007 | G/G | G |
|
|
| chr10:94949129 | A/A | A |
|
||
| chr10:94949144 | C/C | C |
|
||
| chr10:94949145 | rs772782449 | C/C | C |
|
|
| chr10:94949161 | AT/AT | AT |
|
||
| chr10:94949217 | rs2256871 | A/A | A |
|
|
| chr10:94949280 | rs9332130 | A/A | A |
|
|
| chr10:94949281 | rs9332131 | GA/GA | GA |
|
|
| chr10:94972119 | rs182132442 | C/C | C |
|
|
| chr10:94972123 | C/C | C |
|
||
| chr10:94972134 | A/A | A |
|
||
| chr10:94972179 | rs72558192 | A/A | A |
|
|
| chr10:94972180 | rs988617574 | C/C | C |
|
|
| chr10:94972183 | A/A | A |
|
||
| chr10:94972233 | rs1237225311 | C/C | C |
|
|
| chr10:94981199 | G/G | G |
|
||
| chr10:94981201 | rs57505750 | T/T | T |
|
|
| chr10:94981224 | rs28371685 | C/C | C |
|
|
| chr10:94981225 | rs367826293 | G/G | G |
|
|
| chr10:94981230 | rs1274535931 | C/C | C |
|
|
| chr10:94981250 | rs750820937 | C/C | C |
|
|
| chr10:94981258 | rs1297714792 | C/C | C |
|
|
| chr10:94981281 | rs749060448 | G/G | G |
|
|
| chr10:94981296 | rs1057910 | A/A | A |
|
|
| chr10:94981297 | rs56165452 | T/T | T |
|
|
| chr10:94981301 | rs28371686 | C/C | C |
|
|
| chr10:94981302 | rs1250577724 | C/C | C |
|
|
| chr10:94981305 | rs578144976 | C/C | C |
|
|
| chr10:94981365 | C/C | C |
|
||
| chr10:94981371 | rs542577750 | G/G | G |
|
|
| chr10:94986042 | rs764211126 | A/A | A |
|
|
| chr10:94986073 | rs72558193 | A/A | A |
|
|
| chr10:94986136 | rs1254213342 | A/A | A |
|
|
| chr10:94986174 | rs1441296358 | G/G | G |
|
|
| chr10:94988852 | rs776908257 | C/C | C |
|
|
| chr10:94988855 | A/A | A |
|
||
| chr10:94988880 | G/G | G |
|
||
| chr10:94988917 | rs769942899 | G/G | G |
|
|
| chr10:94988925 | rs202201137 | A/A | A |
|
|
| chr10:94988955 | rs767284820 | T/T | T |
|
|
| chr10:94988984 | rs781583846 | G/G | G |
|
|
| chr10:94989020 | rs9332239 | C/C | C |
|
|
| chr10:94989023 | rs868182778 | G/G | G |
|
Other Positions of Interest
| Position in VCF | RSID | Call in VCF |
|---|---|---|
| chr10:94645745 | rs12777823 | G/G |
CYP3A4 allele match data
| Allele Matched: | *1/*1 |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr7:99758183 | rs67666821 | G/G | G |
|
|
| chr7:99758228 | rs1584538410 | T/T | T |
|
|
| chr7:99760836 | rs4986913 | G/G | G |
|
|
| chr7:99760901 | rs4986910 | A/A | A |
|
|
| chr7:99760956 | rs774109750 | T/T | T |
|
|
| chr7:99762047 | rs4986909 | G/G | G |
|
|
| chr7:99762054 | A/A | A |
|
||
| chr7:99762069 | T/T | T |
|
||
| chr7:99762177 | rs12721629 | G/G | G |
|
|
| chr7:99762186 | rs756833413 | C/C | C |
|
|
| chr7:99762206 | rs67784355 | G/G | G |
|
|
| chr7:99762234 | rs1318364992 | C/C | C |
|
|
| chr7:99763877 | rs368296206 | A/A | A |
|
|
| chr7:99763909 | rs1303250043 | G/G | G |
|
|
| chr7:99763925 | rs201821708 | T/T | T |
|
|
| chr7:99764003 | rs28371759 | A/A | A |
|
|
| chr7:99766411 | rs4646438 | G/G | G |
|
|
| chr7:99766424 | rs1429705359 | T/T | T |
|
|
| chr7:99766439 | rs145582851 | C/C | C |
|
|
| chr7:99766440 | rs138105638 | G/G | G |
|
|
| chr7:99768360 | rs55785340 | A/A | A |
|
|
| chr7:99768371 | rs55901263 | G/G | G |
|
|
| chr7:99768424 | rs113667357 | T/T | T |
|
|
| chr7:99768447 | rs3208361 | T/T | T |
|
|
| chr7:99768458 | rs4987161 | A/A | A |
|
|
| chr7:99768470 | rs12721627 | G/G | G |
|
|
| chr7:99768693 | rs35599367 | G/G | G |
|
|
| chr7:99769769 | rs4986908 | C/C | C |
|
|
| chr7:99769781 | rs72552798 | C/C | C |
|
|
| chr7:99769804 | rs4986907 | C/C | C |
|
|
| chr7:99769805 | rs57409622 | G/G | G |
|
|
| chr7:99770165 | rs72552799 | C/C | C |
|
|
| chr7:99770166 | rs778013004 | G/G | G |
|
|
| chr7:99770196 | T/T | T |
|
||
| chr7:99770202 | rs55951658 | T/T | T |
|
|
| chr7:99770217 | rs1449865051 | A/A | A |
|
|
| chr7:99778079 | rs56324128 | C/C | C |
|
|
| chr7:99780036 | G/G | G |
|
||
| chr7:99784018 | rs570051168 | G/G | G |
|
|
| chr7:99784038 | rs12721634 | A/A | A |
|
|
| chr7:99784075 | rs188389063 | G/G | G |
|
|
| chr7:99784078 | rs1226205448 | C/C | C |
|
CYP3A5 allele match data
| Allele Matched: | *1/*1 |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr7:99652770 | rs41303343 | T/T | T |
|
|
| chr7:99660516 | rs28383479 | C/C | C |
|
|
| chr7:99665212 | rs10264272 | C/C | C |
|
|
| chr7:99672916 | rs776746 | T/T | T |
|
|
| chr7:99676198 | rs55817950 | G/G | G |
|
CYP4F2 allele match data
| Allele Matched: | *1/*1 |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr19:15878779 | rs3093200 | G/G | G |
|
|
| chr19:15878920 | rs4020346 | T/T | T |
|
|
| chr19:15879412 | rs138971789 | C/C | C |
|
|
| chr19:15879621 | rs2108622 | C/C | C |
|
|
| chr19:15886018 | rs145174239 | G/G | G |
|
|
| chr19:15889671 | rs144233412 | C/C | C |
|
|
| chr19:15890405 | rs3093153 | C/C | C |
|
|
| chr19:15892541 | rs145875499 | C/C | C |
|
|
| chr19:15895527 | rs114396708 | G/G | G |
|
|
| chr19:15895560 | rs144455532 | G/G | G |
|
|
| chr19:15897466 | rs201380574 | C/C | C |
|
|
| chr19:15897473 | rs115517770 | G/G | G |
|
|
| chr19:15897566 | rs114099324 | C/C | C |
|
|
| chr19:15897578 | rs3093105 | A/A | A |
|
DPYD allele match data
| Allele Matched: | Reference/Reference |
|---|---|
| Phasing Status: |
Unphased |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr1:97078987 | rs114096998 | G/G | G |
|
|
| chr1:97078993 | rs148799944 | C/C | C |
|
|
| chr1:97079005 | rs140114515 | C/C | C |
|
|
| chr1:97079071 | rs1801268 | C/C | C |
|
|
| chr1:97079076 | rs139459586 | A/A | A |
|
|
| chr1:97079077 | rs202144771 | G/G | G |
|
|
| chr1:97079121 | rs72547601 | T/T | T |
|
|
| chr1:97079133 | rs72547602 | T/T | T |
|
|
| chr1:97079139 | rs145529148 | T/T | T |
|
|
| chr1:97082365 | rs141044036 | T/T | T |
|
|
| chr1:97082391 | rs67376798 | T/T | T |
|
|
| chr1:97098598 | rs1801267 | C/C | C |
|
|
| chr1:97098599 | rs147545709 | G/G | G |
|
|
| chr1:97098616 | rs55674432 | C/C | C |
|
|
| chr1:97098632 | rs201035051 | T/T | T |
|
|
| chr1:97193109 | rs60139309 | T/T | T |
|
|
| chr1:97193209 | rs200687447 | C/C | C |
|
|
| chr1:97234958 | rs199634007 | G/G | G |
|
|
| chr1:97234991 | rs56005131 | G/G | G |
|
|
| chr1:97305279 | rs112766203 | G/G | G |
|
|
| chr1:97305363 | rs60511679 | A/A | A |
|
|
| chr1:97305364 | rs1801160 | C/C | C |
|
|
| chr1:97305372 | rs146529561 | G/G | G |
|
|
| chr1:97306195 | rs145548112 | C/C | C |
|
|
| chr1:97373598 | rs137999090 | C/C | C |
|
|
| chr1:97373629 | rs138545885 | C/C | C |
|
|
| chr1:97382461 | rs55971861 | T/T | T |
|
|
| chr1:97450058 | rs3918290 | C/C | C |
|
|
| chr1:97450059 | rs3918289 | G/G | G |
|
|
| chr1:97450065 | rs72549303 | TG/TG | TG |
|
|
| chr1:97450068 | rs17376848 | A/A | A |
|
|
| chr1:97450168 | rs147601618 | A/A | A |
|
|
| chr1:97450187 | rs145773863 | C/C | C |
|
|
| chr1:97450189 | rs138616379 | C/C | C |
|
|
| chr1:97450190 | rs59086055 | G/G | G |
|
|
| chr1:97515784 | rs201615754 | C/C | C |
|
|
| chr1:97515787 | rs55886062 | A/A | A |
|
|
| chr1:97515839 | rs1801159 | T/T | T |
|
|
| chr1:97515851 | rs142619737 | C/C | C |
|
|
| chr1:97515865 | rs1801158 | C/C | C |
|
|
| chr1:97515889 | rs190951787 | G/G | G |
|
|
| chr1:97515923 | rs148994843 | C/C | C |
|
|
| chr1:97549565 | rs138391898 | C/C | C |
|
|
| chr1:97549600 | rs111858276 | T/T | T |
|
|
| chr1:97549609 | rs72549304 | G/G | G |
|
|
| chr1:97549681 | rs199549923 | G/G | G |
|
|
| chr1:97549713 | rs57918000 | G/G | G |
|
|
| chr1:97549726 | rs144395748 | G/G | G |
|
|
| chr1:97549735 | rs72975710 | G/G | G |
|
|
| chr1:97573785 | rs186169810 | A/A | A |
|
|
| chr1:97573805 | rs142512579 | C/C | C |
|
|
| chr1:97573821 | rs764666241 | C/C | C |
|
|
| chr1:97573839 | rs200064537 | A/A | A |
|
|
| chr1:97573863 | rs56038477 | C/C | C |
|
|
| chr1:97573881 | rs61622928 | C/C | C |
|
|
| chr1:97573918 | rs143815742 | C/C | C |
|
|
| chr1:97573919 | rs140602333 | G/G | G |
|
|
| chr1:97573943 | rs78060119 | C/C | C |
|
|
| chr1:97579893 | rs75017182 | G/G | G |
|
|
| chr1:97593238 | rs72549305 | T/T | T |
|
|
| chr1:97593289 | rs143154602 | G/G | G |
|
|
| chr1:97593322 | rs183385770 | C/C | C |
|
|
| chr1:97593343 | rs72549306 | C/C | C |
|
|
| chr1:97593379 | rs201018345 | C/C | C |
|
|
| chr1:97595083 | rs145112791 | G/G | G |
|
|
| chr1:97595088 | rs150437414 | A/A | A |
|
|
| chr1:97595149 | rs146356975 | T/T | T |
|
|
| chr1:97679170 | rs45589337 | T/T | T |
|
|
| chr1:97691776 | rs1801266 | G/G | G |
|
|
| chr1:97699399 | rs72549307 | T/T | T |
|
|
| chr1:97699430 | rs72549308 | T/T | T |
|
|
| chr1:97699474 | rs115232898 | T/T | T |
|
|
| chr1:97699506 | rs6670886 | C/C | C |
|
|
| chr1:97699533 | rs139834141 | C/C | C |
|
|
| chr1:97699535 | rs2297595 | T/T | T |
|
|
| chr1:97721542 | rs200562975 | T/T | T |
|
|
| chr1:97721650 | rs141462178 | T/T | T |
|
|
| chr1:97740400 | rs150385342 | C/C | C |
|
|
| chr1:97740410 | rs72549309 | GATGA/ GATGA |
GATGA |
|
|
| chr1:97883329 | rs1801265 | A/A | A |
|
|
| chr1:97883352 | rs80081766 | C/C | C |
|
|
| chr1:97883353 | rs72549310 | G/G | G |
|
|
| chr1:97883368 | rs150036960 | G/G | G |
|
G6PD allele match data
| Variant Matched: | B (reference)/B (reference) |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chrX:154532046 | A/A | A |
|
||
| chrX:154532055 | CTCT/CTCT | CTCT |
|
||
| chrX:154532082 | G/G | G |
|
||
| chrX:154532083 | G/G | G |
|
||
| chrX:154532085 | C/C | C |
|
||
| chrX:154532086 | C/C | C |
|
||
| chrX:154532203 | rs137852348 | G/G | G |
|
|
| chrX:154532231 | T/T | T |
|
||
| chrX:154532245 | rs137852344 | G/G | G |
|
|
| chrX:154532257 | rs72554664 | C/C | C |
|
|
| chrX:154532258 | G/G | G |
|
||
| chrX:154532264 | rs782608284 | C/C | C |
|
|
| chrX:154532265 | C/C | C |
|
||
| chrX:154532269 | rs72554665 | C/C | C |
|
|
| chrX:154532278 | T/T | T |
|
||
| chrX:154532279 | C/C | C |
|
||
| chrX:154532389 | rs137852324 | C/C | C |
|
|
| chrX:154532390 | rs398123546 | G/G | G |
|
|
| chrX:154532392 | A/A | A |
|
||
| chrX:154532403 | C/C | C |
|
||
| chrX:154532408 | T/T | T |
|
||
| chrX:154532411 | rs137852317 | C/C | C |
|
|
| chrX:154532432 | G/G | G |
|
||
| chrX:154532434 | rs137852337 | C/C | C |
|
|
| chrX:154532458 | A/A | A |
|
||
| chrX:154532459 | rs782098548 | C/C | C |
|
|
| chrX:154532570 | G/G | G |
|
||
| chrX:154532590 | G/G | G |
|
||
| chrX:154532608 | C/C | C |
|
||
| chrX:154532623 | T/T | T |
|
||
| chrX:154532625 | rs137852336 | C/C | C |
|
|
| chrX:154532626 | rs137852323 | C/C | C |
|
|
| chrX:154532628 | G/G | G |
|
||
| chrX:154532629 | G/G | G |
|
||
| chrX:154532634 | T/T | T |
|
||
| chrX:154532639 | C/C | C |
|
||
| chrX:154532649 | G/G | G |
|
||
| chrX:154532661 | T/T | T |
|
||
| chrX:154532662 | rs137852325 | C/C | C |
|
|
| chrX:154532667 | G/G | G |
|
||
| chrX:154532674 | rs137852335 | C/C | C |
|
|
| chrX:154532676 | rs137852316 | C/C | C |
|
|
| chrX:154532677 | G/G | G |
|
||
| chrX:154532679 | A/A | A |
|
||
| chrX:154532688 | T/T | T |
|
||
| chrX:154532692 | T/T | T |
|
||
| chrX:154532694 | rs137852321 | C/C | C |
|
|
| chrX:154532695 | rs137852334 | G/G | G |
|
|
| chrX:154532698 | rs137852320 | T/T | T |
|
|
| chrX:154532699 | G/G | G |
|
||
| chrX:154532700 | C/C | C |
|
||
| chrX:154532701 | rs137852322 | A/A | A |
|
|
| chrX:154532713 | A/A | A |
|
||
| chrX:154532715 | A/A | A |
|
||
| chrX:154532716 | T/T | T |
|
||
| chrX:154532722 | rs371489738 | C/C | C |
|
|
| chrX:154532752 | CGGCCTTGC GCTCGTTCA G/ CGGCCTTGC GCTCGTTCA G |
CGGCCTTGC GCTCGTTCA G |
|
||
| chrX:154532758 | T/T | T |
|
||
| chrX:154532765 | rs137852329 | G/G | G |
|
|
| chrX:154532772 | rs137852345 | G/G | G |
|
|
| chrX:154532773 | C/C | C |
|
||
| chrX:154532797 | rs137852333 | G/G | G |
|
|
| chrX:154532802 | C/C | C |
|
||
| chrX:154532945 | rs34193178 | C/C | C |
|
|
| chrX:154532956 | rs398123544 | T/T | T |
|
|
| chrX:154532969 | rs137852342 | G/G | G |
|
|
| chrX:154532987 | T/T | T |
|
||
| chrX:154532989 | G/G | G |
|
||
| chrX:154532990 | rs5030869 | C/C | C |
|
|
| chrX:154533004 | C/C | C |
|
||
| chrX:154533012 | CGTGGGGTC GTCCAGGTA CCCTTTG/ CGTGGGGTC GTCCAGGTA CCCTTTG |
CGTGGGGTC GTCCAGGTA CCCTTTG |
|
||
| chrX:154533016 | G/G | G |
|
||
| chrX:154533025 | rs76723693 | A/A | A |
|
|
| chrX:154533029 | rs137852347 | A/A | A |
|
|
| chrX:154533031 | C/C | C |
|
||
| chrX:154533044 | rs137852339 | C/C | C |
|
|
| chrX:154533064 | C/C | C |
|
||
| chrX:154533072 | C/C | C |
|
||
| chrX:154533077 | C/C | C |
|
||
| chrX:154533083 | C/C | C |
|
||
| chrX:154533122 | rs137852327 | C/C | C |
|
|
| chrX:154533586 | rs74575103 | C/C | C |
|
|
| chrX:154533587 | G/G | G |
|
||
| chrX:154533589 | A/A | A |
|
||
| chrX:154533591 | G/G | G |
|
||
| chrX:154533592 | T/T | T |
|
||
| chrX:154533596 | rs137852318 | C/C | C |
|
|
| chrX:154533605 | T/T | T |
|
||
| chrX:154533607 | G/G | G |
|
||
| chrX:154533608 | A/A | A |
|
||
| chrX:154533614 | G/G | G |
|
||
| chrX:154533615 | C/C | C |
|
||
| chrX:154533619 | T/T | T |
|
||
| chrX:154533620 | C/C | C |
|
||
| chrX:154533629 | C/C | C |
|
||
| chrX:154533634 | rs137852346 | C/C | C |
|
|
| chrX:154534036 | G/G | G |
|
||
| chrX:154534074 | TCAGTGC/ TCAGTGC |
TCAGTGC |
|
||
| chrX:154534092 | T/T | T |
|
||
| chrX:154534102 | rs782757170 | G/G | G |
|
|
| chrX:154534110 | C/C | C |
|
||
| chrX:154534116 | ATGT/ATGT | ATGT |
|
||
| chrX:154534125 | rs137852328 | C/C | C |
|
|
| chrX:154534126 | G/G | G |
|
||
| chrX:154534157 | rs137852319 | A/A | A |
|
|
| chrX:154534345 | rs137852326 | C/C | C |
|
|
| chrX:154534348 | rs782754619 | T/T | T |
|
|
| chrX:154534387 | rs781865768 | T/T | T |
|
|
| chrX:154534389 | rs137852332 | C/C | C |
|
|
| chrX:154534390 | rs137852330 | G/G | G |
|
|
| chrX:154534409 | G/G | G |
|
||
| chrX:154534414 | GGGA/GGGA | GGGA |
|
||
| chrX:154534419 | rs5030868 | G/G | G |
|
|
| chrX:154534438 | rs267606836 | G/G | G |
|
|
| chrX:154534440 | rs5030872 | T/T | T |
|
|
| chrX:154534447 | T/T | T |
|
||
| chrX:154534455 | T/T | T |
|
||
| chrX:154534463 | G/G | G |
|
||
| chrX:154534465 | rs137852343 | A/A | A |
|
|
| chrX:154534468 | G/G | G |
|
||
| chrX:154534485 | C/C | C |
|
||
| chrX:154534486 | G/G | G |
|
||
| chrX:154534489 | rs137852331 | T/T | T |
|
|
| chrX:154534494 | C/C | C |
|
||
| chrX:154534495 | rs137852314 | C/C | C |
|
|
| chrX:154535176 | rs370918918 | C/C | C |
|
|
| chrX:154535180 | rs782487723 | C/C | C |
|
|
| chrX:154535187 | rs137852313 | C/C | C |
|
|
| chrX:154535190 | G/G | G |
|
||
| chrX:154535211 | C/C | C |
|
||
| chrX:154535244 | G/G | G |
|
||
| chrX:154535247 | G/G | G |
|
||
| chrX:154535249 | rs782322505 | T/T | T |
|
|
| chrX:154535261 | C/C | C |
|
||
| chrX:154535269 | G/G | G |
|
||
| chrX:154535270 | rs78365220 | A/A | A |
|
|
| chrX:154535274 | C/C | C |
|
||
| chrX:154535277 | rs1050829 | T/T | T |
|
|
| chrX:154535278 | C/C | C |
|
||
| chrX:154535301 | A/A | A |
|
||
| chrX:154535316 | rs5030870 | C/C | C |
|
|
| chrX:154535330 | A/A | A |
|
||
| chrX:154535336 | rs267606835 | G/G | G |
|
|
| chrX:154535342 | rs181277621 | C/C | C |
|
|
| chrX:154535367 | GCTT/GCTT | GCTT |
|
||
| chrX:154535379 | G/G | G |
|
||
| chrX:154535962 | rs782308266 | C/C | C |
|
|
| chrX:154535963 | rs138687036 | G/G | G |
|
|
| chrX:154535980 | A/A | A |
|
||
| chrX:154535995 | rs782090947 | T/T | T |
|
|
| chrX:154535996 | rs137852349 | A/A | A |
|
|
| chrX:154536002 | rs1050828 | C/C | C |
|
|
| chrX:154536008 | A/A | A |
|
||
| chrX:154536019 | G/G | G |
|
||
| chrX:154536021 | CAGA/CAGA | CAGA |
|
||
| chrX:154536025 | A/A | A |
|
||
| chrX:154536032 | rs137852315 | C/C | C |
|
|
| chrX:154536034 | C/C | C |
|
||
| chrX:154536035 | G/G | G |
|
||
| chrX:154536045 | C/C | C |
|
||
| chrX:154536151 | G/G | G |
|
||
| chrX:154536156 | rs76645461 | A/A | A |
|
|
| chrX:154536168 | rs78478128 | G/G | G |
|
|
| chrX:154536169 | C/C | C |
|
||
| chrX:154546045 | rs137852338 | CATG/CATG | CATG |
|
|
| chrX:154546046 | A/A | A |
|
||
| chrX:154546057 | T/T | T |
|
||
| chrX:154546061 | rs137852340 | T/T | T |
|
|
| chrX:154546116 | C/C | C |
|
||
| chrX:154546122 | C/C | C |
|
||
| chrX:154546131 | G/G | G |
|
IFNL3/4 allele match data
| Allele Matched: | rs12979860 reference (C)/rs12979860 reference (C) |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr19:39248147 | rs12979860 | C/C | C |
|
NUDT15 allele match data
| Allele Matched: | *1/*1 |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr13:48037748 | rs769369441 | T/T | T |
|
|
| chr13:48037749 | G/G | G |
|
||
| chr13:48037782 | rs746071566 | AGGAGTC/ AGGAGTC |
AGGAGTC |
|
|
| chr13:48037798 | rs186364861 | G/G | G |
|
|
| chr13:48037825 | rs777311140 | C/C | C |
|
|
| chr13:48037834 | rs1202487323 | C/C | C |
|
|
| chr13:48037847 | rs766023281 | G/G | G |
|
|
| chr13:48037849 | A/A | A |
|
||
| chr13:48037885 | rs1950545307 | G/G | G |
|
|
| chr13:48037902 | rs149436418 | C/C | C |
|
|
| chr13:48040977 | rs1457579126 | GA/GA | GA |
|
|
| chr13:48041103 | rs761191455 | T/T | T |
|
|
| chr13:48041113 | rs1368252918 | G/G | G |
|
|
| chr13:48045690 | rs768324690 | C/C | C |
|
|
| chr13:48045714 | rs1185228044 | G/G | G |
|
|
| chr13:48045719 | rs116855232 | C/C | C |
|
|
| chr13:48045720 | rs147390019 | G/G | G |
|
|
| chr13:48045771 | rs139551410 | T/T | T |
|
RYR1 allele match data
| Allele Matched: | Reference/Reference |
|---|---|
| Phasing Status: |
Unphased |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr19:38433867 | rs193922744 | T/T | T |
|
|
| chr19:38440747 | rs193922745 | CGAT/CGAT | CGAT |
|
|
| chr19:38440796 | rs193922746 | A/A | A |
|
|
| chr19:38440802 | rs193922747 | T/T | T |
|
|
| chr19:38440818 | G/G | G |
|
||
| chr19:38440829 | rs193922748 | C/C | C |
|
|
| chr19:38440830 | rs139161723 | G/G | G |
|
|
| chr19:38440851 | rs193922749 | C/C | C |
|
|
| chr19:38442361 | rs118192160 | G/G | G |
|
|
| chr19:38442373 | rs995399438 | T/T | T |
|
|
| chr19:38442395 | rs118192113 | C/C | C |
|
|
| chr19:38442434 | rs186983396 | C/C | C |
|
|
| chr19:38443790 | rs142474192 | G/G | G |
|
|
| chr19:38444179 | C/C | C |
|
||
| chr19:38444187 | rs193922750 | C/C | C |
|
|
| chr19:38444191 | rs193922751 | G/G | G |
|
|
| chr19:38444203 | rs193922752 | A/A | A |
|
|
| chr19:38444211 | rs118192161 | C/C | C |
|
|
| chr19:38444212 | rs193922753 | G/G | G |
|
|
| chr19:38444217 | rs193922754 | G/G | G |
|
|
| chr19:38444220 | rs193922755 | G/G | G |
|
|
| chr19:38444221 | rs193922756 | A/A | A |
|
|
| chr19:38444250 | rs761616815 | G/G | G |
|
|
| chr19:38444252 | G/G | G |
|
||
| chr19:38444253 | rs193922757 | C/C | C |
|
|
| chr19:38444257 | A/A | A |
|
||
| chr19:38444671 | rs771058055 | G/G | G |
|
|
| chr19:38446481 | rs727504129 | C/C | C |
|
|
| chr19:38446492 | rs193922759 | G/G | G |
|
|
| chr19:38446517 | rs112596687 | T/T | T |
|
|
| chr19:38446520 | rs193922760 | A/A | A |
|
|
| chr19:38446710 | rs1801086 | G/G | G |
|
|
| chr19:38448500 | rs752652072 | C/C | C |
|
|
| chr19:38448501 | rs193922761 | G/G | G |
|
|
| chr19:38448673 | rs193922762 | C/C | C |
|
|
| chr19:38448680 | T/T | T |
|
||
| chr19:38448712 | rs121918592 | G/G | G |
|
|
| chr19:38448715 | G/G | G |
|
||
| chr19:38448791 | rs113332073 | G/G | G |
|
|
| chr19:38451785 | C/C | C |
|
||
| chr19:38451842 | rs193922764 | C/C | C |
|
|
| chr19:38451843 | rs193922766 | G/G | G |
|
|
| chr19:38451850 | rs118192116 | C/C | C |
|
|
| chr19:38452985 | C/C | C |
|
||
| chr19:38452996 | rs193922767 | G/G | G |
|
|
| chr19:38455247 | rs147723844 | A/A | A |
|
|
| chr19:38455253 | C/C | C |
|
||
| chr19:38455254 | T/T | T |
|
||
| chr19:38455269 | rs901087791 | G/G | G |
|
|
| chr19:38455347 | T/T | T |
|
||
| chr19:38455359 | rs118192162 | A/A | A |
|
|
| chr19:38455463 | rs111888148 | G/G | G |
|
|
| chr19:38455471 | rs193922768 | C/C | C |
|
|
| chr19:38455472 | rs144336148 | G/G | G |
|
|
| chr19:38455489 | rs193922769 | T/T | T |
|
|
| chr19:38455504 | G/G | G |
|
||
| chr19:38455528 | rs193922770 | C/C | C |
|
|
| chr19:38457539 | rs118204423 | G/G | G |
|
|
| chr19:38457545 | rs118192172 | C/C | C |
|
|
| chr19:38457546 | rs193922772 | G/G | G |
|
|
| chr19:38458175 | rs747177274 | G/G | G |
|
|
| chr19:38458247 | rs138874610 | G/G | G |
|
|
| chr19:38460461 | rs376149732 | C/C | C |
|
|
| chr19:38460551 | rs193922775 | C/C | C |
|
|
| chr19:38463499 | rs370634440 | G/G | G |
|
|
| chr19:38464649 | rs148623597 | G/G | G |
|
|
| chr19:38466144 | rs778241277 | G/G | G |
|
|
| chr19:38466216 | rs180714609 | G/G | G |
|
|
| chr19:38466315 | rs141942845 | G/G | G |
|
|
| chr19:38466347 | rs111272095 | C/C | C |
|
|
| chr19:38466386 | rs2145447772 | G/G | G |
|
|
| chr19:38466392 | G/G | G |
|
||
| chr19:38467655 | rs749040743 | G/G | G |
|
|
| chr19:38469002 | rs193922776 | C/C | C |
|
|
| chr19:38469111 | rs549201486 | C/C | C |
|
|
| chr19:38469404 | A/A | A |
|
||
| chr19:38469415 | rs936513262 | G/G | G |
|
|
| chr19:38473635 | rs34694816 | A/A | A |
|
|
| chr19:38475335 | rs137933390 | A/A | A |
|
|
| chr19:38477816 | rs145573319 | A/A | A |
|
|
| chr19:38483293 | rs146429605 | A/A | A |
|
|
| chr19:38483329 | rs754476250 | C/C | C |
|
|
| chr19:38483345 | rs151029675 | C/C | C |
|
|
| chr19:38483357 | rs193922777 | C/C | C |
|
|
| chr19:38485679 | T/T | T |
|
||
| chr19:38485688 | rs781104539 | A/A | A |
|
|
| chr19:38485691 | rs146504767 | G/G | G |
|
|
| chr19:38485787 | rs754785770 | A/A | A |
|
|
| chr19:38485838 | rs193922781 | C/C | C |
|
|
| chr19:38485841 | rs193922782 | T/T | T |
|
|
| chr19:38485972 | rs192863857 | C/C | C |
|
|
| chr19:38485996 | rs372958050 | T/T | T |
|
|
| chr19:38486015 | rs34934920 | C/C | C |
|
|
| chr19:38486095 | A/A | A |
|
||
| chr19:38486096 | rs193922783 | T/T | T |
|
|
| chr19:38490151 | rs145801146 | C/C | C |
|
|
| chr19:38490642 | A/A | A |
|
||
| chr19:38492540 | rs35364374 | G/G | G |
|
|
| chr19:38494379 | rs746818096 | T/T | T |
|
|
| chr19:38494381 | rs770593660 | G/G | G |
|
|
| chr19:38494426 | rs193922788 | G/G | G |
|
|
| chr19:38494454 | G/G | G |
|
||
| chr19:38494464 | rs117886618 | C/C | C |
|
|
| chr19:38494465 | rs193922789 | G/G | G |
|
|
| chr19:38494555 | rs143398211 | G/G | G |
|
|
| chr19:38494564 | rs118192175 | C/C | C |
|
|
| chr19:38494565 | rs118192163 | G/G | G |
|
|
| chr19:38494579 | rs118192176 | G/G | G |
|
|
| chr19:38494621 | rs193922790 | A/A | A |
|
|
| chr19:38494625 | rs959170123 | G/G | G |
|
|
| chr19:38496265 | rs193922791 | C/C | C |
|
|
| chr19:38496278 | rs141646642 | C/C | C |
|
|
| chr19:38496283 | rs118192177 | C/C | C |
|
|
| chr19:38496294 | rs193922792 | G/G | G |
|
|
| chr19:38496301 | rs193922793 | T/T | T |
|
|
| chr19:38496306 | rs193922795 | G/G | G |
|
|
| chr19:38496415 | rs199870223 | C/C | C |
|
|
| chr19:38496416 | rs537994744 | G/G | G |
|
|
| chr19:38496455 | G/G | G |
|
||
| chr19:38496487 | rs763352221 | C/C | C |
|
|
| chr19:38496488 | rs140152019 | G/G | G |
|
|
| chr19:38496502 | rs917523269 | C/C | C |
|
|
| chr19:38496901 | rs193922797 | G/G | G |
|
|
| chr19:38496910 | rs118192121 | A/A | A |
|
|
| chr19:38499177 | rs34390345 | A/A | A |
|
|
| chr19:38499223 | rs112563513 | G/G | G |
|
|
| chr19:38499234 | T/T | T |
|
||
| chr19:38499241 | rs147213895 | A/A | A |
|
|
| chr19:38499639 | rs193922798 | G/G | G |
|
|
| chr19:38499642 | C/C | C |
|
||
| chr19:38499643 | rs193922799 | G/G | G |
|
|
| chr19:38499644 | rs121918596 | TGGA/TGGA | TGGA |
|
|
| chr19:38499650 | rs193922801 | A/A | A |
|
|
| chr19:38499655 | rs193922802 | G/G | G |
|
|
| chr19:38499667 | G/G | G |
|
||
| chr19:38499670 | rs193922803 | C/C | C |
|
|
| chr19:38499680 | T/T | T |
|
||
| chr19:38499682 | rs769482889 | C/C | C |
|
|
| chr19:38499683 | G/G | G |
|
||
| chr19:38499691 | rs762401851 | G/G | G |
|
|
| chr19:38499692 | rs193922804 | A/A | A |
|
|
| chr19:38499696 | C/C | C |
|
||
| chr19:38499697 | rs193922805 | T/T | T |
|
|
| chr19:38499704 | rs193922806 | C/C | C |
|
|
| chr19:38499706 | rs146306934 | G/G | G |
|
|
| chr19:38499719 | A/A | A |
|
||
| chr19:38499730 | G/G | G |
|
||
| chr19:38499731 | rs193922807 | G/G | G |
|
|
| chr19:38499806 | rs976108591 | A/A | A |
|
|
| chr19:38499817 | rs111364296 | G/G | G |
|
|
| chr19:38499975 | rs193922809 | G/G | G |
|
|
| chr19:38499984 | rs193922810 | G/G | G |
|
|
| chr19:38499985 | A/A | A |
|
||
| chr19:38499993 | rs121918593 | G/G | G |
|
|
| chr19:38499997 | rs28933396 | G/G | G |
|
|
| chr19:38500000 | G/G | G |
|
||
| chr19:38500003 | rs193922812 | C/C | C |
|
|
| chr19:38500010 | rs193922813 | G/G | G |
|
|
| chr19:38500636 | rs118192124 | C/C | C |
|
|
| chr19:38500637 | rs193922815 | G/G | G |
|
|
| chr19:38500640 | rs118192123 | T/T | T |
|
|
| chr19:38500642 | rs193922816 | C/C | C |
|
|
| chr19:38500643 | rs118192122 | G/G | G |
|
|
| chr19:38500654 | rs28933397 | C/C | C |
|
|
| chr19:38500655 | rs121918594 | G/G | G |
|
|
| chr19:38500667 | rs551223467 | C/C | C |
|
|
| chr19:38500863 | rs193922817 | C/C | C |
|
|
| chr19:38500898 | rs118192178 | C/C | C |
|
|
| chr19:38500899 | rs193922818 | G/G | G |
|
|
| chr19:38500904 | rs193922819 | T/T | T |
|
|
| chr19:38502652 | rs767553612 | A/A | A |
|
|
| chr19:38502663 | rs193922822 | C/C | C |
|
|
| chr19:38502669 | C/C | C |
|
||
| chr19:38502670 | rs751180702 | G/G | G |
|
|
| chr19:38502679 | rs193922824 | C/C | C |
|
|
| chr19:38502708 | rs748575133 | T/T | T |
|
|
| chr19:38502923 | rs914804033 | G/G | G |
|
|
| chr19:38504298 | G/G | G |
|
||
| chr19:38504319 | rs193922826 | C/C | C |
|
|
| chr19:38504347 | rs781126470 | C/C | C |
|
|
| chr19:38504868 | rs193922827 | G/G | G |
|
|
| chr19:38504869 | rs112196644 | A/A | A |
|
|
| chr19:38504878 | rs193922828 | G/G | G |
|
|
| chr19:38505061 | rs193922829 | G/G | G |
|
|
| chr19:38505325 | rs147707463 | C/C | C |
|
|
| chr19:38505358 | rs35180584 | C/C | C |
|
|
| chr19:38505923 | rs193922830 | C/C | C |
|
|
| chr19:38505932 | rs768535909 | T/T | T |
|
|
| chr19:38506361 | rs193922831 | T/T | T |
|
|
| chr19:38506492 | rs112772310 | G/G | G |
|
|
| chr19:38506508 | C/C | C |
|
||
| chr19:38506865 | C/C | C |
|
||
| chr19:38507821 | C/C | C |
|
||
| chr19:38511590 | rs147303895 | G/G | G |
|
|
| chr19:38512279 | G/G | G |
|
||
| chr19:38512321 | rs193922832 | G/G | G |
|
|
| chr19:38512367 | rs193922833 | G/G | G |
|
|
| chr19:38515052 | C/C | C |
|
||
| chr19:38516167 | rs199738299 | A/A | A |
|
|
| chr19:38516181 | T/T | T |
|
||
| chr19:38516184 | rs553055844 | G/G | G |
|
|
| chr19:38516208 | G/G | G |
|
||
| chr19:38517431 | rs375626634 | T/T | T |
|
|
| chr19:38517470 | T/T | T |
|
||
| chr19:38517521 | rs757753317 | G/G | G |
|
|
| chr19:38517523 | T/T | T |
|
||
| chr19:38517541 | rs112151058 | G/G | G |
|
|
| chr19:38519237 | rs118204421 | C/C | C |
|
|
| chr19:38519238 | rs193922834 | G/G | G |
|
|
| chr19:38519292 | rs137932199 | G/G | G |
|
|
| chr19:38519295 | rs118192126 | A/A | A |
|
|
| chr19:38519424 | C/C | C |
|
||
| chr19:38519432 | A/A | A |
|
||
| chr19:38519447 | A/A | A |
|
||
| chr19:38525432 | C/C | C |
|
||
| chr19:38525492 | rs143987857 | G/G | G |
|
|
| chr19:38527707 | rs55876273 | G/G | G |
|
|
| chr19:38527710 | G/G | G |
|
||
| chr19:38528372 | G/G | G |
|
||
| chr19:38529002 | G/G | G |
|
||
| chr19:38529036 | rs193922838 | G/G | G |
|
|
| chr19:38529042 | C/C | C |
|
||
| chr19:38529048 | rs375915752 | C/C | C |
|
|
| chr19:38534726 | rs4802584 | C/C | C |
|
|
| chr19:38534774 | rs763112609 | C/C | C |
|
|
| chr19:38534775 | rs193922839 | G/G | G |
|
|
| chr19:38535197 | rs111565359 | G/G | G |
|
|
| chr19:38535998 | rs140616359 | G/G | G |
|
|
| chr19:38543365 | rs148399313 | G/G | G |
|
|
| chr19:38543380 | A/A | A |
|
||
| chr19:38543405 | rs193922840 | T/T | T |
|
|
| chr19:38543551 | rs147136339 | A/A | A |
|
|
| chr19:38543566 | G/G | G |
|
||
| chr19:38543810 | C/C | C |
|
||
| chr19:38543816 | rs118204422 | T/T | T |
|
|
| chr19:38543821 | rs193922842 | C/C | C |
|
|
| chr19:38543832 | rs193922843 | G/G | G |
|
|
| chr19:38546460 | rs755088027 | G/G | G |
|
|
| chr19:38546496 | rs773040531 | A/A | A |
|
|
| chr19:38548253 | A/A | A |
|
||
| chr19:38548259 | rs144685735 | C/C | C |
|
|
| chr19:38548287 | rs193922844 | C/C | C |
|
|
| chr19:38548380 | rs373406011 | C/C | C |
|
|
| chr19:38561140 | G/G | G |
|
||
| chr19:38561185 | rs193922848 | A/A | A |
|
|
| chr19:38561213 | C/C | C |
|
||
| chr19:38561228 | rs201321695 | A/A | A |
|
|
| chr19:38561236 | rs193922849 | C/C | C |
|
|
| chr19:38561243 | rs193922850 | T/T | T |
|
|
| chr19:38561362 | G/G | G |
|
||
| chr19:38561363 | G/G | G |
|
||
| chr19:38561383 | rs151119428 | G/G | G |
|
|
| chr19:38565023 | T/T | T |
|
||
| chr19:38565034 | rs193922852 | G/G | G |
|
|
| chr19:38565182 | rs193922853 | A/A | A |
|
|
| chr19:38565215 | rs587784372 | C/C | C |
|
|
| chr19:38565218 | rs193922855 | C/C | C |
|
|
| chr19:38566978 | rs139647387 | A/A | A |
|
|
| chr19:38566986 | rs150396398 | G/G | G |
|
|
| chr19:38570619 | rs771741606 | C/C | C |
|
|
| chr19:38570620 | rs118192130 | G/G | G |
|
|
| chr19:38570649 | C/C | C |
|
||
| chr19:38572032 | rs143520367 | C/C | C |
|
|
| chr19:38572185 | rs118192135 | G/G | G |
|
|
| chr19:38572190 | rs756850145 | A/A | A |
|
|
| chr19:38572206 | rs193922860 | G/G | G |
|
|
| chr19:38572262 | rs759500310 | T/T | T |
|
|
| chr19:38572266 | rs193922862 | TC/TC | TC |
|
|
| chr19:38573180 | rs193922863 | C/C | C |
|
|
| chr19:38573229 | rs193922864 | T/T | T |
|
|
| chr19:38573304 | rs118192140 | C/C | C |
|
|
| chr19:38575957 | rs200766617 | G/G | G |
|
|
| chr19:38577931 | A/A | A |
|
||
| chr19:38577942 | rs193922865 | T/T | T |
|
|
| chr19:38577946 | rs193922866 | G/G | G |
|
|
| chr19:38577954 | rs193922867 | C/C | C |
|
|
| chr19:38577955 | rs193922868 | G/G | G |
|
|
| chr19:38578015 | rs768360593 | G/G | G |
|
|
| chr19:38578205 | G/G | G |
|
||
| chr19:38580004 | rs118192167 | A/A | A |
|
|
| chr19:38580039 | TT/TT | TT |
|
||
| chr19:38580041 | C/C | C |
|
||
| chr19:38580066 | rs143988412 | A/A | A |
|
|
| chr19:38580075 | rs193922873 | G/G | G |
|
|
| chr19:38580088 | rs193922874 | T/T | T |
|
|
| chr19:38580094 | rs121918595 | C/C | C |
|
|
| chr19:38580114 | rs193922876 | C/C | C |
|
|
| chr19:38580126 | C/C | C |
|
||
| chr19:38580370 | rs193922878 | C/C | C |
|
|
| chr19:38580382 | rs193922879 | G/G | G |
|
|
| chr19:38580397 | G/G | G |
|
||
| chr19:38580403 | rs118192168 | G/G | G |
|
|
| chr19:38580416 | C/C | C |
|
||
| chr19:38580425 | rs193922880 | C/C | C |
|
|
| chr19:38580439 | rs118192181 | C/C | C |
|
|
| chr19:38580440 | rs63749869 | G/G | G |
|
|
| chr19:38580485 | rs113210953 | A/A | A |
|
|
| chr19:38580497 | rs193922883 | T/T | T |
|
|
| chr19:38584974 | rs118192151 | G/G | G |
|
|
| chr19:38584976 | rs193922888 | G/G | G |
|
|
| chr19:38584989 | rs118192170 | T/T | T |
|
|
| chr19:38585078 | A/A | A |
|
||
| chr19:38585099 | G/G | G |
|
||
| chr19:38585947 | rs111657878 | T/T | T |
|
|
| chr19:38585948 | rs118192159 | C/C | C |
|
|
| chr19:38585951 | rs193922895 | C/C | C |
|
|
| chr19:38585952 | rs118192158 | G/G | G |
|
|
| chr19:38585959 | rs193922896 | G/G | G |
|
|
| chr19:38586101 | rs193922898 | T/T | T |
|
|
| chr19:38586140 | rs146876145 | C/C | C |
|
|
| chr19:38586190 | A/A | A |
|
||
| chr19:38587362 | G/G | G |
|
||
| chr19:38587363 | G/G | G |
|
SLCO1B1 allele match data
| Allele Matched: | *1/*1 |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
In case no genotype can be determined, recommendations are based on the rs4149056 genotype alone as per guideline. The minor C allele at rs4149056 defines SLCO1B1*5.
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr12:21172734 | rs139257324 | C/C | C |
|
|
| chr12:21172776 | rs373327528 | G/G | G |
|
|
| chr12:21172782 | rs56101265 | T/T | T |
|
|
| chr12:21174595 | rs56061388 | T/T | T |
|
|
| chr12:21176804 | rs2306283 | A/A | A |
|
|
| chr12:21176868 | rs2306282 | A/A | A |
|
|
| chr12:21176871 | G/G | G |
|
||
| chr12:21176879 | rs11045819 | C/C | C |
|
|
| chr12:21176883 | rs72559745 | A/A | A |
|
|
| chr12:21176898 | rs77271279 | G/G | G |
|
|
| chr12:21178612 | rs141467543 | A/A | A |
|
|
| chr12:21178615 | rs4149056 | T/T | T |
|
|
| chr12:21178957 | rs79135870 | A/A | A |
|
|
| chr12:21196951 | rs11045852 | A/A | A |
|
|
| chr12:21196975 | rs183501729 | C/C | C |
|
|
| chr12:21196976 | rs11045853 | G/G | G |
|
|
| chr12:21200544 | rs72559747 | C/C | C |
|
|
| chr12:21200595 | rs55901008 | T/T | T |
|
|
| chr12:21202553 | rs1228465562 | T/T | T |
|
|
| chr12:21202555 | rs59113707 | C/C | C |
|
|
| chr12:21202649 | rs56387224 | A/A | A |
|
|
| chr12:21202664 | rs142965323 | G/G | G |
|
|
| chr12:21205921 | rs72559748 | A/A | A |
|
|
| chr12:21205999 | rs59502379 | G/G | G |
|
|
| chr12:21206031 | rs74064213 | A/A | A |
|
|
| chr12:21222355 | rs71581941 | C/C | C |
|
|
| chr12:21239042 | rs34671512 | A/A | A |
|
|
| chr12:21239077 | rs56199088 | A/A | A |
|
|
| chr12:21239113 | rs55737008 | A/A | A |
|
|
| chr12:21239145 | rs200995543 | C/C | C |
|
|
| chr12:21239158 | rs140790673 | C/C | C |
|
TPMT allele match data
| Allele Matched: | *1/*1 |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr6:18130687 | rs1142345 | T/T | T |
|
|
| chr6:18130694 | rs150900439 | T/T | T |
|
|
| chr6:18130725 | rs72552736 | A/A | A |
|
|
| chr6:18130729 | rs139392616 | C/C | C |
|
|
| chr6:18130758 | rs398122996 | A/A | A |
|
|
| chr6:18130762 | rs56161402 | C/C | C |
|
|
| chr6:18130772 | rs377085266 | A/A | A |
|
|
| chr6:18130781 | rs1800584 | C/C | C |
|
|
| chr6:18132136 | rs72556347 | A/A | A |
|
|
| chr6:18132147 | rs79901429 | A/A | A |
|
|
| chr6:18132163 | C/C | C |
|
||
| chr6:18133845 | rs75543815 | T/T | T |
|
|
| chr6:18133847 | rs6921269 | C/C | C |
|
|
| chr6:18133870 | rs772832951 | A/A | A |
|
|
| chr6:18133884 | rs74423290 | G/G | G |
|
|
| chr6:18133887 | rs201695576 | T/T | T |
|
|
| chr6:18133890 | rs9333570 | C/C | C |
|
|
| chr6:18138969 | rs144041067 | C/C | C |
|
|
| chr6:18138970 | rs112339338 | G/G | G |
|
|
| chr6:18138997 | rs1800460 | C/C | C |
|
|
| chr6:18139027 | rs72552737 | C/C | C |
|
|
| chr6:18139689 | rs72552738 | C/C | C |
|
|
| chr6:18139710 | rs200220210 | G/G | G |
|
|
| chr6:18143597 | T/T | T |
|
||
| chr6:18143606 | rs151149760 | T/T | T |
|
|
| chr6:18143613 | C/C | C |
|
||
| chr6:18143622 | rs115106679 | C/C | C |
|
|
| chr6:18143643 | A/A | A |
|
||
| chr6:18143700 | rs753545734 | C/C | C |
|
|
| chr6:18143718 | rs111901354 | G/G | G |
|
|
| chr6:18143724 | rs1800462 | C/C | C |
|
|
| chr6:18143728 | rs1256618794 | C/C | C |
|
|
| chr6:18147838 | rs281874771 | G/G | G |
|
|
| chr6:18147845 | rs777686348 | C/C | C |
|
|
| chr6:18147851 | rs200591577 | G/G | G |
|
|
| chr6:18147856 | A/A | A |
|
||
| chr6:18147910 | rs72552740 | A/A | A |
|
|
| chr6:18149004 | G/G | G |
|
||
| chr6:18149022 | rs750424422 | C/C | C |
|
|
| chr6:18149032 | rs759836180 | C/C | C |
|
|
| chr6:18149045 | rs72552742 | T/T | T |
|
|
| chr6:18149126 | rs267607275 | A/A | A |
|
|
| chr6:18149127 | rs9333569 | T/T | T |
|
UGT1A1 allele match data
| Allele Matched: | *1/*1 |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr2:233759924 | rs887829 | C/C | C |
|
|
| chr2:233760233 | rs3064744 | CAT/CAT | CAT |
|
|
| chr2:233760498 | rs4148323 | G/G | G |
|
|
| chr2:233760973 | rs35350960 | C/C | C |
|
VKORC1 allele match data
| Variant Matched: | rs9923231 reference (C)/rs9923231 reference (C) |
|---|---|
| Phasing Status: |
Unphased PharmCAT reports the genotype(s) that receive the highest score during the matcher process. In case of unphased data, additional genotypes might be possible and cannot be ruled out. |
Calls at Positions
| Position in VCF | RSID | Call in VCF | Reference | Related Alleles and Function | Warnings |
|---|---|---|---|---|---|
| chr16:31096368 | rs9923231 | C/C | C |
|
Disclaimers and Other Information
Liability: PharmCAT assumes no responsibility for any injury to person or damage to persons or property arising out of, or related to any use of PharmCAT, or for any errors or omissions. The user recognizes that PharmCAT is a research tool and that they are using PharmCAT at their own risk.
A. Allele and Genotype Determination
PharmCAT uses gene allele definitions included in the CPIC database, with exceptions as noted in Gene Definition Exceptions document. For allele definitions and the positions used in PharmCAT, see the gene definition tables.
Genes with DPWG recommendations that are not included in CPIC are discussed in Section C.
PharmCAT results are dependent on the supplied VCF calls for the queried positions (for technical information about PharmCAT input formatting and requirements, please go to pharmcat.org). PharmCAT does not assume any reference calls for positions missing from the submitted VCF file; all missing queried positions are not considered in the allele determination process. See the pharmcat_positions file for which positions are queried in the VCF file. Missing positions might alter the assigned genotype and subsequent phenotype prediction and CPIC recommendation. If the supplied VCF file has missing positions, those positions will be noted in Section III: Allele Matching Details for each gene of this report. For the most reliable allele determination, reference calls as well as variant calls in the VCF file for every queried position must be provided by the user. If an allele that includes a missing position is defined by an additional position(s) for which calls are provided, the allele will be considered in the matching process based on the available information. This might lead to the output of multiple possible genotypes that received the same highest matching score. In addition, alternate calls with a lower score is also possible. For instructions on getting PharmCAT to output all possible matching genotypes, consult the documentation.
For cytochrome P450 genes, TPMT, NUDT15, UGT1A1, and SLCO1B1, the *1 allele is defined by the absence of variation specified in the gene definition tables. This allele cannot be identified by variants; rather, *1 is assigned by default when no variation for the queried positions is reported in the submitted VCF file. The same is true for all other genes with multiple variant positions in the definition table (CACNA1S, CFTR, DPYD, RYR1): the reference sequence is the default result when variants are not reported in the VCF file. It is always possible un-interrogated variation can occur which could potentially affect allele function, but because it is undetected, the assignment would be defaulted to a *1 (or reference) allele and normal function.
For all genes, variation reported in the VCF file but NOT included in the gene definition table will not be considered during allele assignment. There is a possibility that any such variation results in a reduced or no activity allele which could lead to inaccurate phenotype and CPIC recommendation, similar to the situation in point 3, above.
Nucleotide base calls are displayed on the positive chromosomal strand regardless of the gene strand; further information is provided under Gene-specific warnings in Section III: Allele Matching Details.
Structural variation star alleles that cannot be detected using VCF file data:
- CYP2B7-CYP2B6 hybrids: CYP2B6*29, CYP2B6*30
- Partial and whole gene deletions: CYP2C19*36, CYP2C19*37, CYP4F2*16, SLCO1B1*48, SLCO1B1*49
PharmCAT matches variants to genotypes assuming unphased data (unless phased data is provided in the VCF file and noted as such, see pharmcat.org for details). The assumption is that defined alleles exist in trans configuration, i.e. on opposite chromosomes, with exceptions noted in Section III: Allele Matching Details under "Gene-specific warnings." However, in cases where an allele is defined by a combination of two or more variants, where each variant alone also defines an allele, the match is based on the longer allele. For example, TPMT*3B is defined by one SNP, *3C is defined by another SNP, and *3A is defined by the combination of those two SNPs. In the case of unphased data that is heterozygous for both SNPs, the *1/*3A genotype is returned though the possibility of *3B/*3C cannot be ruled out.
Below cases are summarized for which two calls with different scores are possible when provided unphased data and heterozygous calls for the variants that define the two alleles. The genotype with the higher score (longer allele) will be used to determine allele functionality, phenotype, and recommendation but the genotype with the lower score cannot be ruled out.
Table 1: Cases for which there is an overlap in the allele definitions.
| Gene | Genotype (Higher Score) | Phenotype | Genotype (Lower Score) | Phenotype |
|---|---|---|---|---|
| UGT1A1 | *1/*80+*28 | Intermediate | *28/*80 | Indeterminate |
| UGT1A1 | *1/*80+*37 | Intermediate | *37/*80 | Indeterminate |
| TPMT | *1/*3A | Intermediate | *3B/*3C | Poor |
| NUDT15 | *1/*2 | Intermediate | *3/*6 | Possible Intermediate |
| CYP2C9 | *1/*71 | N/A | *10/*22 | Indeterminate |
| CYP2B6 | *1/*36 | Intermediate | *6/*22 | Intermediate |
| CYP2B6 | *1/*34 | Intermediate | *33/*36 | Indeterminate |
| CYP2B6 | *1/*6 | Intermediate | *4/*9 | Intermediate |
| CYP2B6 | *1/*7 | Intermediate | *5/*6 | Intermediate |
| CYP2B6 | *1/*13 | Intermediate | *6/*8 | Intermediate |
| SLCO1B1 | *1/*46 | Decreased function | *15/*45 | Possible Decreased Function |
| SLCO1B1 | *1/*20 | Normal Function | *19/*37 | Indeterminate |
| SLCO1B1 | *1/*12 | Indeterminate | *2/*10 | Indeterminate |
| SLCO1B1 | *1/*13 | Indeterminate | *3/*11 | Indeterminate |
| SLCO1B1 | *1/*14 | Normal Function | *4/*37 | Indeterminate |
| SLCO1B1 | *1/*15 | Decreased function | *5/*37 | Decreased function |
| SLCO1B1 | *1/*25 | Indeterminate | *4/*28 | Indeterminate |
| SLCO1B1 | *1/*31 | Decreased function | *9/*37 | Decreased Function |
| SLCO1B1 | *1/*32 | Indeterminate | *4/*24 | Indeterminate |
| SLCO1B1 | *1/*40 | Indeterminate | *5/*19 | Possible Decreased Function |
| SLCO1B1 | *1/*43 | Indeterminate | *4/*44 | Indeterminate |
| CYP4F2 | *1/*4 | N/A | *2/*3 | N/A |
| CYP3A4 | *1/*37 | N/A | *3/*22 | N/A |
| CYP3A4 | *1/*38 | N/A | *3/*11 | N/A |
| G6PD | A- 202A_376G/B (reference) | Variable | A/Asahi | Variable |
| G6PD | B (reference)/Mt Sinai | Variable | A/Guadalajara | Variable |
| G6PD | B (reference)/Santa Maria | Variable | A/Malaga | Variable |
| G6PD | Ananindeua/B (reference) | Variable | A/Viangchan, Jammu | Variable |
| G6PD | B (reference)/Hechi | Variable | Asahi/Viangchan, Jammu | Deficient |
| G6PD | B (reference)/Hermoupolis | Variable | Cassano/Union,Maewo, Chinese-2, Kalo | Deficient |
Table 2: Cases for which there is an overlap in the allele definitions because the definition of the non-*1 allele in the genotype with the higher score allows for reference or variant at the position that defines the first allele listed in the genotype with the lower score. Both genotypes cannot be ruled out with unphased data if the position that overlaps between the respective alleles is heterozygous (0/1) in addition to heterozygous calls for the other variants that define the non-*1 allele in the genotype with the higher score.
| Gene | Genotype (Higher Score) | Phenotype | Genotype (Lower Score) | Phenotype |
|---|---|---|---|---|
| CYP2C19 | *1/*4 | Intermediate | *17/*4 | Intermediate |
| CYP2C19 | *1/*2 | Intermediate | *11/*2 | Intermediate |
| CYP2C19 | *1/*35 | Intermediate | *15/*35 | Intermediate |
| CYP2B6 | *1/*18 | Intermediate | *4/*18 | Indeterminate |
B. CPIC Allele Function, Phenotype and Recommendation
All content is sourced from the CPIC database.
C. DPWG Allele Function, Phenotype and Recommendation
PharmGKB annotates PGx-based drug dosing guidelines published by the Royal Dutch Association for the Advancement of Pharmacy - Pharmacogenetics Working Group (DPWG). PharmGKB curates allele function assignments and phenotype mappings from the DPWG to provide genotype specific DPWG guideline recommendations. Where possible, PharmGKB maps DPWG terms to CPIC terms, as outlined on PharmGKB.
CYP3A4 is currently not part of a CPIC guideline. Since the DPWG CYP3A4 documentation includes limit variant notations for the included alleles (only *16, *20, and *22 are specified) PharmCAT relies on PharmVar CYP3A4 allele definitions. However, the CYP3A4*16, *20 and *22 definitions are the same in both sources.
The CPIC UGT1A1 allele definition file includes *6, *27, *28, *36, *37, and *80. Since the DPWG UGT1A1 document does not include allele definitions besides for the UGT1A1 TA box promoter polymorphism, PharmCAT only includes the UGT1A1 positions from the CPIC UGT1A1 allele definition file. Other UGT1A1 alleles can be supplied as outside calls but not be determined from the VCF file by the Named Allele Matcher.
D. FDA drug-label and Table of Pharmacogenetic Associations recommendations
PharmCAT includes recommendations from PharmGKB-annotated FDA drug labels and the FDA Table of Pharmacogenetic Associations. It only contains FDA information for genes with CPIC or DPWG guidelines because the FDA does not offer any genotype-to-phenotype mapping information. PharmCAT uses CPIC genotype-to-phenotype mappings when they exist, and DPWG genotype-to-phenotype mappings when no CPIC mappings exist, to determine the phenotypes to use with FDA label annotations and Table of Pharmacogenetic Associations entries. Results presented for FDA Table of Pharmacogenetic Associations use wording and “Affected Subgroups” taken directly from the Table. Results presented from PharmGKB-annotated drug labels present wording from the specific label that was curated. In many cases, multiple FDA labels may exist for a particular medication. Typically, PharmGKB annotates the label linked to from the FDA Table of Pharmacogenomic Biomarkers in Drug Labeling but in some cases a different label may be annotated. Follow the “FDA Label Annotation” link in section II of the report to access the annotated label and more information.
E. Source Tags in Section II
Part of PharmGKB’s annotations of guidelines, drug labels and Table of Pharmacogenetic Associations is the assignment of labels that are meant to provide a high-level indication of the provided action. If recommendations or FDA Table wording exists, yellow boxes display if the guidance/wording is regarding (1) a dosing change, (2) recommending an alternate drug be used, or (3) other guidance. These boxes are included in the Source column in Section II of the PharmCAT report. More detailed information about what the yellow boxes mean is available on PharmGKB.
F. PharmCAT Exceptions to the CPIC Guideline Gene List
PharmCAT does not determine CYP2D6, MT-RNR1, HLA-A, or HLA-B genotypes from the VCF file, but genotypes for CYP2D6, MT-RNR1, HLA-A, or HLA-B can be provided to PharmCAT from an outside source and the corresponding phenotype prescribing recommendations will be included in the generated report. For the required format of the outside calls refer to the PharmCAT outside calls documentation.
CPIC has assigned function to the following CYP2D6 CNV alleles: *1x2, *1x≥3, *2x2, *2x≥3, *3x2, *4x2, *4x≥3, *6x2, *9x2, *10x2, *17x2, *29x2, *35x2, *36x2, *41x2, *41x3, *43x2, *45x2, *146x2. These alleles are part of the CPIC diplotype to phenotype translation and can be connected to recommendations. Other CNV notations from outside calls need to be mapped accordingly.
G. CPIC Guideline Disclaimers and Caveats
A version of the following quoted disclaimer is part of each CPIC guideline and applies to the CPIC recommendations as used in PharmCAT. For the full description of potential benefits and risks, additional considerations (general and specific to gene-drug pairs), limitations, information about respective gene nomenclature systems, potential drug-drug interactions and clinical factors to consider, please see individual CPIC guidelines at (cpicpgx.org).
"CPIC guidelines reflect expert consensus based on clinical evidence and peer-reviewed literature available at the time they are written and are intended only to assist clinicians in decision-making and to identify questions for further research. New evidence may have emerged since the time a guideline was submitted for publication. Guidelines are limited in scope and are not applicable to interventions or diseases not specifically identified. Guidelines do not account for all individual variations among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It remains the responsibility of the health-care provider to determine the best course of treatment for a patient. Adherence to any guidelines is voluntary, with the ultimate determination regarding its application to be made solely by the clinician and the patient. CPIC assumes no responsibility for any injury to persons or damage to persons or property arising out of or related to any use of CPIC's guidelines, or for any errors or omissions." (PMID: 27997040)
"Caveats: appropriate use and/or potential misuse of genetic tests. The application of genotype-based dosing is most appropriate when initiating therapy with a tricyclic. Obtaining a pharmacogenetic test after months of drug therapy may be less helpful in some instances, as the drug dose may have already been adjusted based on plasma concentrations, response, or side effects. Similar to all diagnostic tests, genetic tests are one of several pieces of clinical information that should be considered before initiating drug therapy." (PMID: 27997040)
CPIC guidelines reflect the alleles/genotypes known and considered by the guideline authors for inclusion by the time of publication, however they may be updated online at cpicpgx.org and in the CPIC database in between publications. Additional alleles and/or more extensive allele definitions might exist by the representative gene nomenclatures for various genes.
CPIC is a registered service mark of the U.S. Department of Health & Human Services (HHS).
H. PharmGKB Disclaimers and Caveats
PharmGKB is a registered service mark of the U.S. Department of Health & Human Services (HHS).